Fig.2D. Ventrobasal thalamus
Evgenii O. Tretiakov
2023-08-17
Last updated: 2023-08-17
Checks: 7 0
Knit directory: Hevesi_2023/
This reproducible R Markdown analysis was created with workflowr (version 1.7.0). The Checks tab describes the reproducibility checks that were applied when the results were created. The Past versions tab lists the development history.
Great! Since the R Markdown file has been committed to the Git repository, you know the exact version of the code that produced these results.
Great job! The global environment was empty. Objects defined in the global environment can affect the analysis in your R Markdown file in unknown ways. For reproduciblity it’s best to always run the code in an empty environment.
The command set.seed(20230121) was run prior to running
the code in the R Markdown file. Setting a seed ensures that any results
that rely on randomness, e.g. subsampling or permutations, are
reproducible.
Great job! Recording the operating system, R version, and package versions is critical for reproducibility.
Nice! There were no cached chunks for this analysis, so you can be confident that you successfully produced the results during this run.
Great job! Using relative paths to the files within your workflowr project makes it easier to run your code on other machines.
Great! You are using Git for version control. Tracking code development and connecting the code version to the results is critical for reproducibility.
The results in this page were generated with repository version 54541d8. See the Past versions tab to see a history of the changes made to the R Markdown and HTML files.
Note that you need to be careful to ensure that all relevant files for
the analysis have been committed to Git prior to generating the results
(you can use wflow_publish or
wflow_git_commit). workflowr only checks the R Markdown
file, but you know if there are other scripts or data files that it
depends on. Below is the status of the Git repository when the results
were generated:
Ignored files:
Ignored: .DS_Store
Ignored: .Rhistory
Ignored: .Rproj.user/
Ignored: .cache/
Ignored: .config/
Ignored: .nv/
Ignored: .snakemake/
Ignored: analysis/figure/
Ignored: cellbender/
Ignored: cellranger/
Ignored: data/Pr5P7_clusters.h5ad
Ignored: data/THP7_clusters.h5ad
Ignored: fastq/
Ignored: souporcell/
Note that any generated files, e.g. HTML, png, CSS, etc., are not included in this status report because it is ok for generated content to have uncommitted changes.
These are the previous versions of the repository in which changes were
made to the R Markdown (analysis/figure_2d-THP7.Rmd) and
HTML (docs/figure_2d-THP7.html) files. If you’ve configured
a remote Git repository (see ?wflow_git_remote), click on
the hyperlinks in the table below to view the files as they were in that
past version.
| File | Version | Author | Date | Message |
|---|---|---|---|---|
| Rmd | 54541d8 | Evgenii O. Tretiakov | 2023-08-17 | updated output |
| html | 9014631 | Evgenii O. Tretiakov | 2023-08-17 | Build site. |
| Rmd | d1dcfea | Evgenii O. Tretiakov | 2023-08-02 | alternative stacked violin plot may be helpful for ventrobasal thalamus where we show two clusters with dotplot relative scale |
| Rmd | edcb936 | Evgenii O. Tretiakov | 2023-03-14 | update methods |
| html | 263f215 | Evgenii O. Tretiakov | 2023-03-13 | Build site. |
| Rmd | 3111dee | Evgenii O. Tretiakov | 2023-03-13 | workflowr::wflow_publish(c("analysis/eda.Rmd", "analysis/figure_2d-THP7.Rmd", |
| html | 4ef209f | Evgenii O. Tretiakov | 2023-03-05 | Build site. |
| Rmd | e584a94 | Evgenii O. Tretiakov | 2023-03-04 | workflowr::wflow_publish(c("analysis/eda.Rmd", "analysis/figure_2d-THP7.Rmd", |
Load dataset
srt <- LoadH5Seurat(here(data_dir, "THP7_clusters.h5Seurat"))
Idents(srt) <- "sep_clstr"
neurons <- subset(srt, subset = Rbfox3 > 0 | Elavl4 > 0 | Snap25 > 0 | Stmn2 > 0)
gneurons <- subset(neurons, subset = Gal > 0)We check list of genes based on our prior knowledge.
gene_of_interest <-
c("Gal", "Galr1", "Galr2", "Galr3",
"Slc17a7", "Slc6a4", "Ache",
"Prkaca", "Adcy1", "Grin1",
"Cck", "Oxt", "Npy", "Sst", "Avp")
genes_present <- Gene_Present(data = srt, gene_list = gene_of_interest)
genes_present$found_features
[1] "Gal" "Galr1" "Galr2" "Slc17a7" "Slc6a4" "Ache" "Prkaca"
[8] "Adcy1" "Grin1" "Cck" "Npy" "Sst"
$bad_features
[1] "Galr3" "Oxt" "Avp"
$wrong_case_found_features
[1] "NA (check not performed. Set 'case_check = TRUE' to perform check."DotPlot_scCustom(
seurat_object = srt,
assay = "RNA",
features = genes_present$found_features,
flip_axes = TRUE,
x_lab_rotate = TRUE,
colors_use = viridis(n = 30, alpha = .75, direction = -1, option = "E"))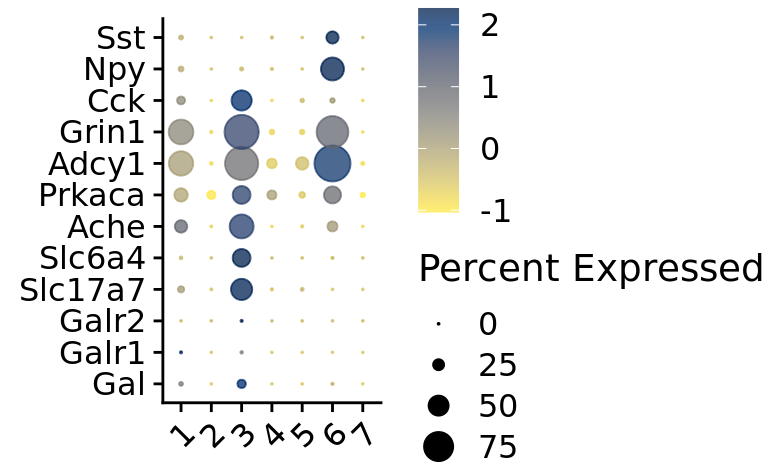
DimPlot_scCustom(
srt,
label = TRUE,
repel = TRUE,
pt.size = 2,
figure_plot = TRUE
) + ggtitle("Clusters") + NoLegend()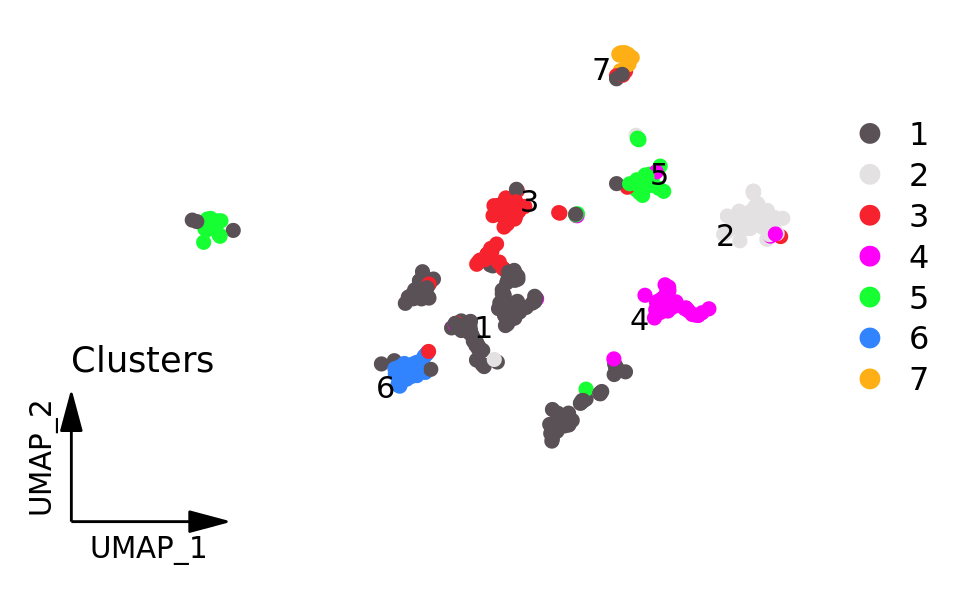
| Version | Author | Date |
|---|---|---|
| 4ef209f | Evgenii O. Tretiakov | 2023-03-05 |
Derive and filter matrix of neurons
mtx_neurons <-
gneurons %>%
GetAssayData("data", "RNA") %>%
as.data.frame() %>%
t()
rownames(mtx_neurons) <- colnames(gneurons)
# Filter features
filt_low_genes <-
colSums(mtx_neurons) %>%
.[. > quantile(., 0.4)] %>%
names()
mtx_neurons %<>% .[, filt_low_genes]
min_filt_vector <-
mtx_neurons %>%
as_tibble() %>%
select(all_of(filt_low_genes)) %>%
summarise(across(.fns = ~ quantile(.x, .1))) %>%
as.list %>%
map(as.double) %>%
simplify %>%
.[colnames(mtx_neurons)]
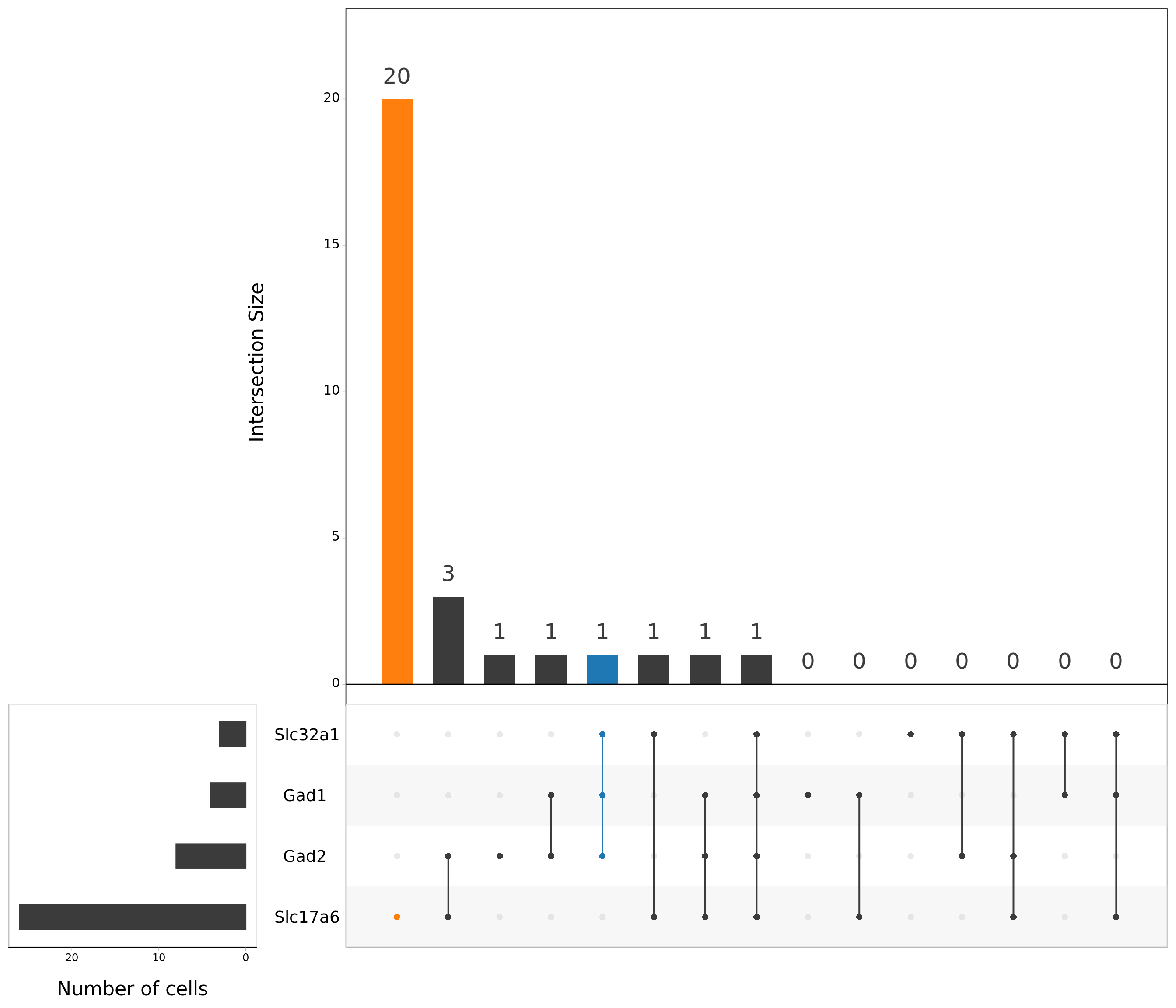
# Prepare table of intersection sets analysis
content_mtx_neuro <-
(mtx_neurons > min_filt_vector) %>%
as_tibble() %>%
mutate_all(as.numeric)Visualise intersections sets that we are going to use (highlighted)
upset(as.data.frame(content_mtx_neuro),
order.by = "freq",
sets.x.label = "Number of cells",
text.scale = c(2, 1.6, 2, 1.3, 2, 3),
nsets = 15,
sets = c("Gad1", "Gad2", "Slc32a1", "Slc17a6"),
queries = list(
list(
query = intersects,
params = list("Gad1", "Gad2", "Slc32a1"),
active = T
),
list(
query = intersects,
params = list("Slc17a6"),
active = T
)
),
nintersects = 60,
empty.intersections = "on"
)
| Version | Author | Date |
|---|---|---|
| 263f215 | Evgenii O. Tretiakov | 2023-03-13 |
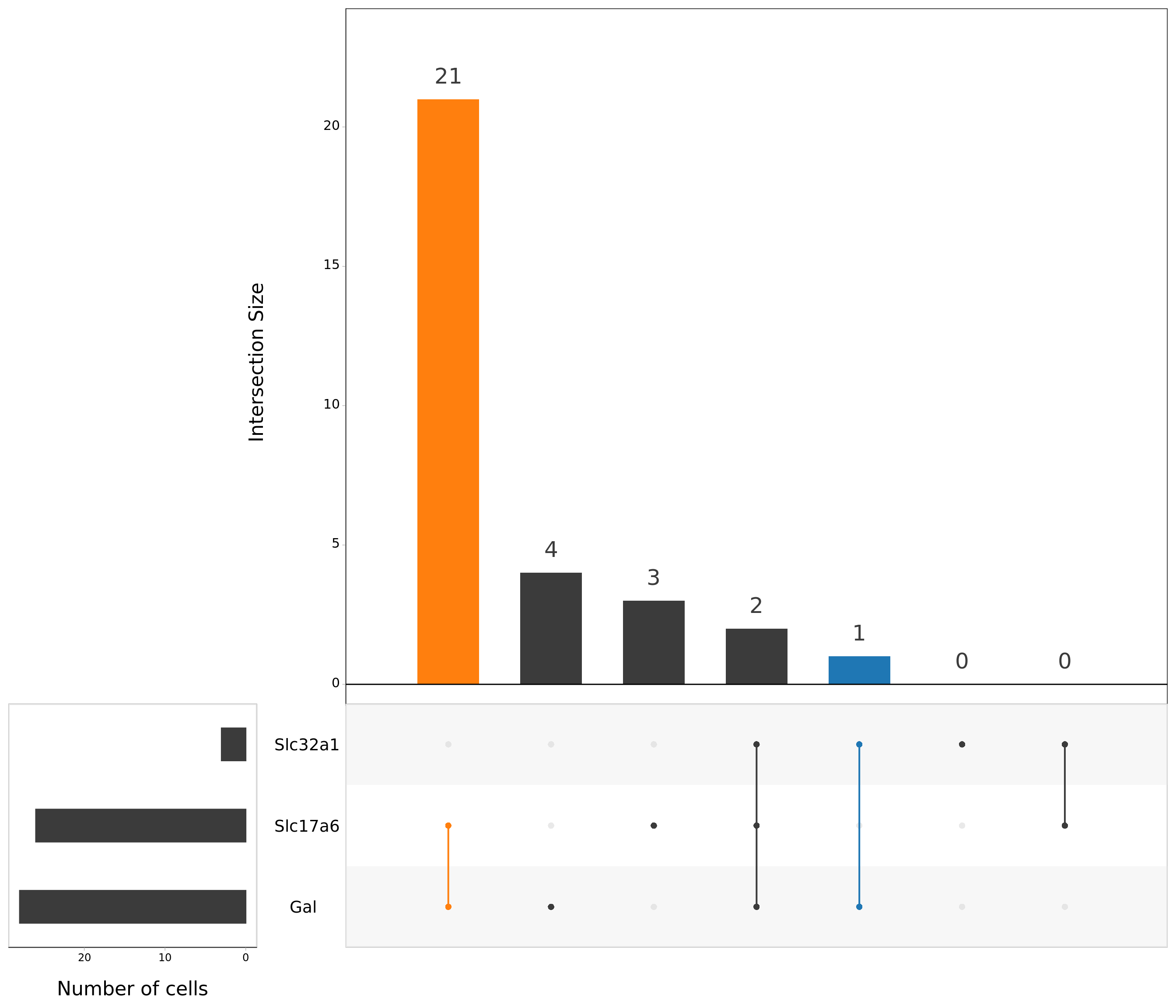
upset(as.data.frame(content_mtx_neuro),
order.by = "freq",
sets.x.label = "Number of cells",
text.scale = c(2, 1.6, 2, 1.3, 2, 3),
nsets = 15,
sets = c("Gal", "Slc32a1", "Slc17a6"),
queries = list(
list(
query = intersects,
params = list("Gal", "Slc32a1"),
active = T
),
list(
query = intersects,
params = list("Gal", "Slc17a6"),
active = T
)
),
nintersects = 60,
empty.intersections = "on"
)
| Version | Author | Date |
|---|---|---|
| 263f215 | Evgenii O. Tretiakov | 2023-03-13 |
Make subset of stable neurons
gneurons$gaba_status <-
content_mtx_neuro %>%
select(Gad1, Gad2, Slc32a1) %>%
mutate(gaba = if_all(.fns = ~ .x > 0)) %>%
.$gaba
gneurons$gaba_expr <-
content_mtx_neuro %>%
select(Gad1, Gad2, Slc32a1) %>%
mutate(gaba = if_any(.fns = ~ .x > 0)) %>%
.$gaba
gneurons$glut_status <-
content_mtx_neuro %>%
select(Slc17a6) %>%
mutate(glut = Slc17a6 > 0) %>%
.$glut
neuro_fin <-
subset(gneurons,
cells = union(
WhichCells(gneurons,
expression = gaba_status == TRUE & glut_status == FALSE),
WhichCells(gneurons,
expression = glut_status == TRUE & gaba_expr == FALSE)))Check contingency tables for neurotransmitter signature
neuro_fin@meta.data %>%
janitor::tabyl(glut_status, gaba_status)Make splits of neurons by neurotransmitter signature
neuro_fin$status <- neuro_fin$gaba_status %>%
if_else(true = "GABAergic",
false = "glutamatergic")
Idents(neuro_fin) <- "status"
SaveH5Seurat(
object = neuro_fin,
filename = here(data_dir, "neuro_fin-THP7"),
overwrite = TRUE,
verbose = TRUE
)
## Split on basis of neurotrans and test for difference
neuro_fin_neurotrans <- SplitObject(neuro_fin, split.by = "status")DotPlots grouped by orig.ident
Expression of GABA receptors in GABAergic Gal positive cells
DotPlot(object = neuro_fin_neurotrans$GABAergic,
features = gabar,
group.by = "orig.ident",
cols = c("#adffff", "#0084ff"),
col.min = -1, col.max = 1
) + RotatedAxis()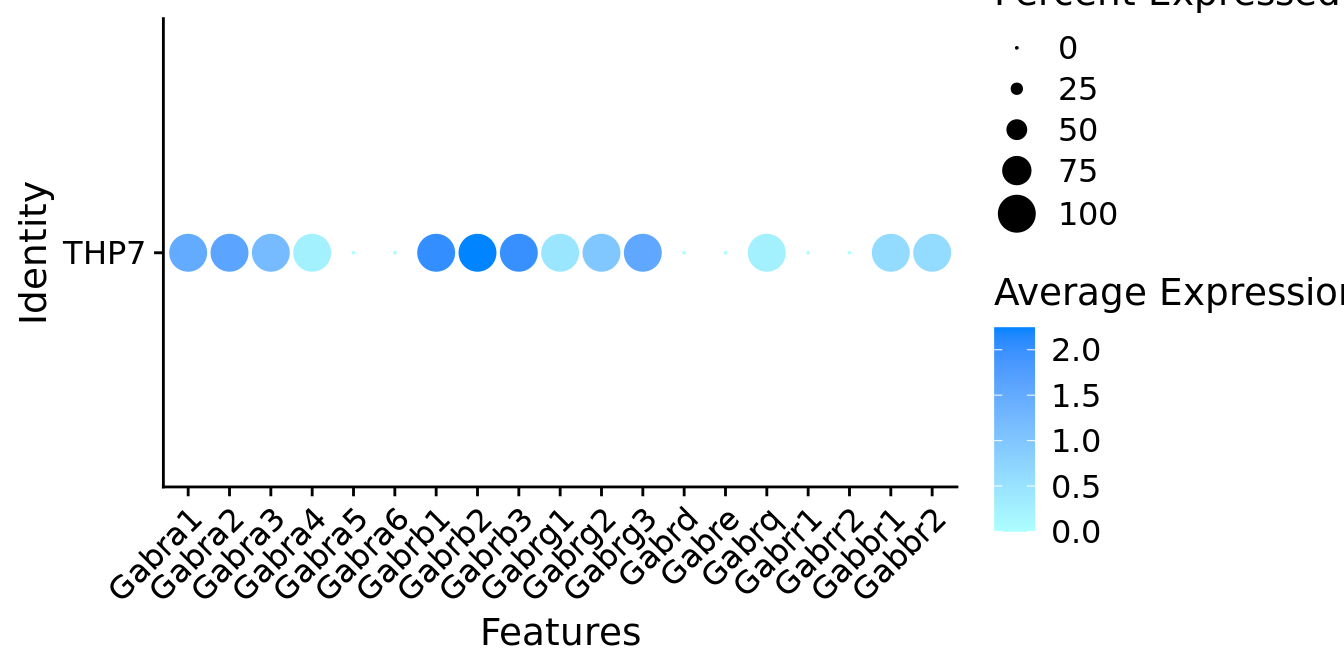
| Version | Author | Date |
|---|---|---|
| 263f215 | Evgenii O. Tretiakov | 2023-03-13 |
Expression of GABA receptors in glutamatergic Gal positive cells
DotPlot(object = neuro_fin_neurotrans$glutamatergic,
features = gabar,
group.by = "orig.ident",
cols = c("#ffc2c2", "#ff3c00"),
col.min = -1, col.max = 1
) + RotatedAxis()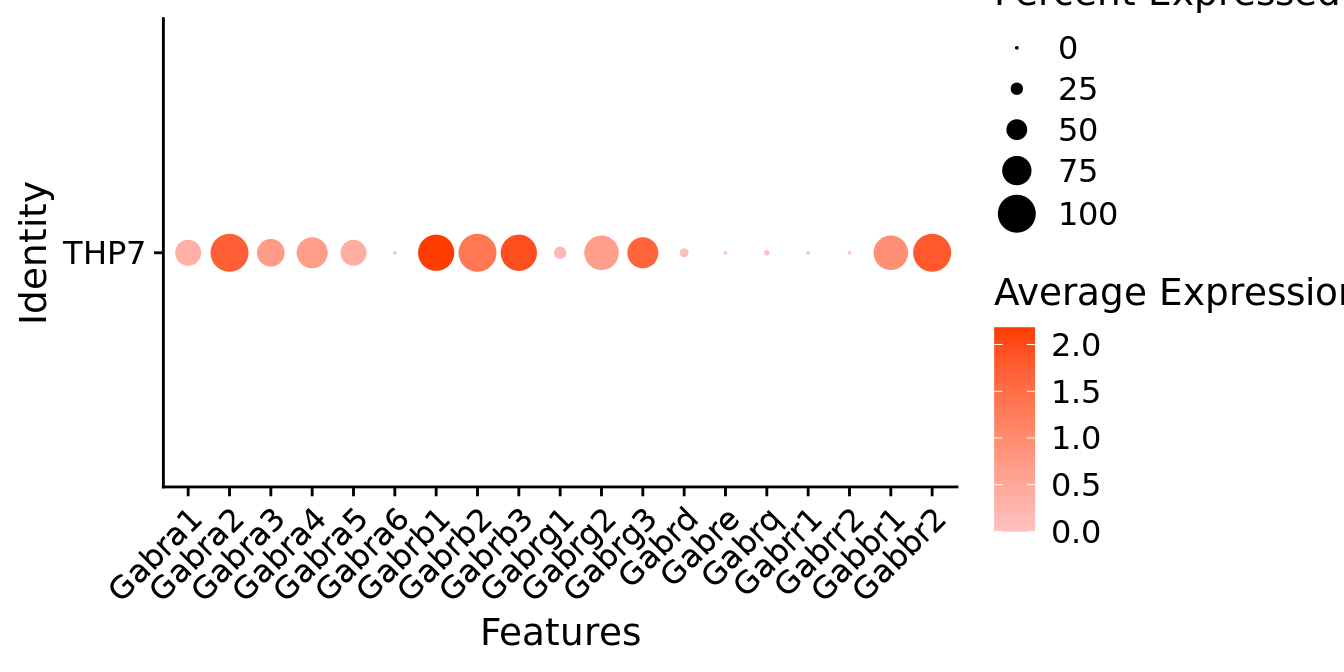
| Version | Author | Date |
|---|---|---|
| 263f215 | Evgenii O. Tretiakov | 2023-03-13 |
Expression of glutamate receptors in GABAergic Gal positive cells
DotPlot(object = neuro_fin_neurotrans$GABAergic,
features = glutr,
group.by = "orig.ident",
cols = c("#adffff", "#0084ff"),
col.min = -1, col.max = 1
) + RotatedAxis()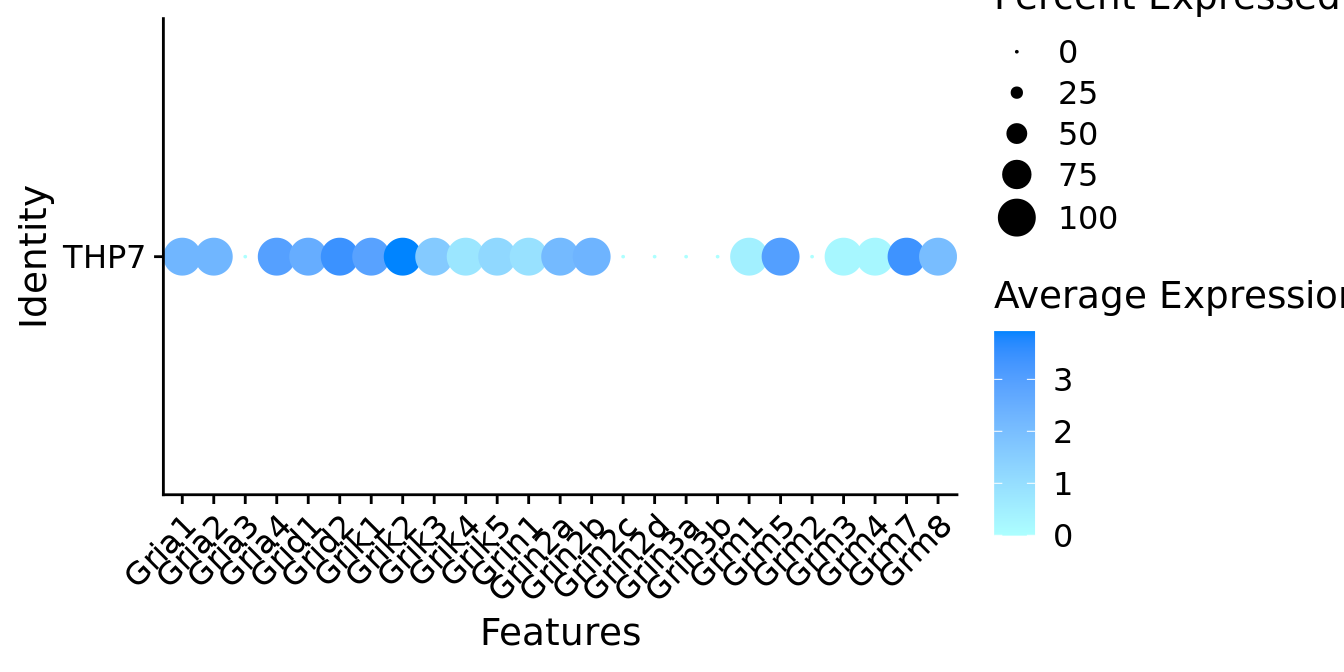
| Version | Author | Date |
|---|---|---|
| 263f215 | Evgenii O. Tretiakov | 2023-03-13 |
Expression of glutamate receptors in glutamatergic Gal positive cells
DotPlot(object = neuro_fin_neurotrans$glutamatergic,
features = glutr,
group.by = "orig.ident",
cols = c("#ffc2c2", "#ff3c00"),
col.min = -1, col.max = 1
) + RotatedAxis()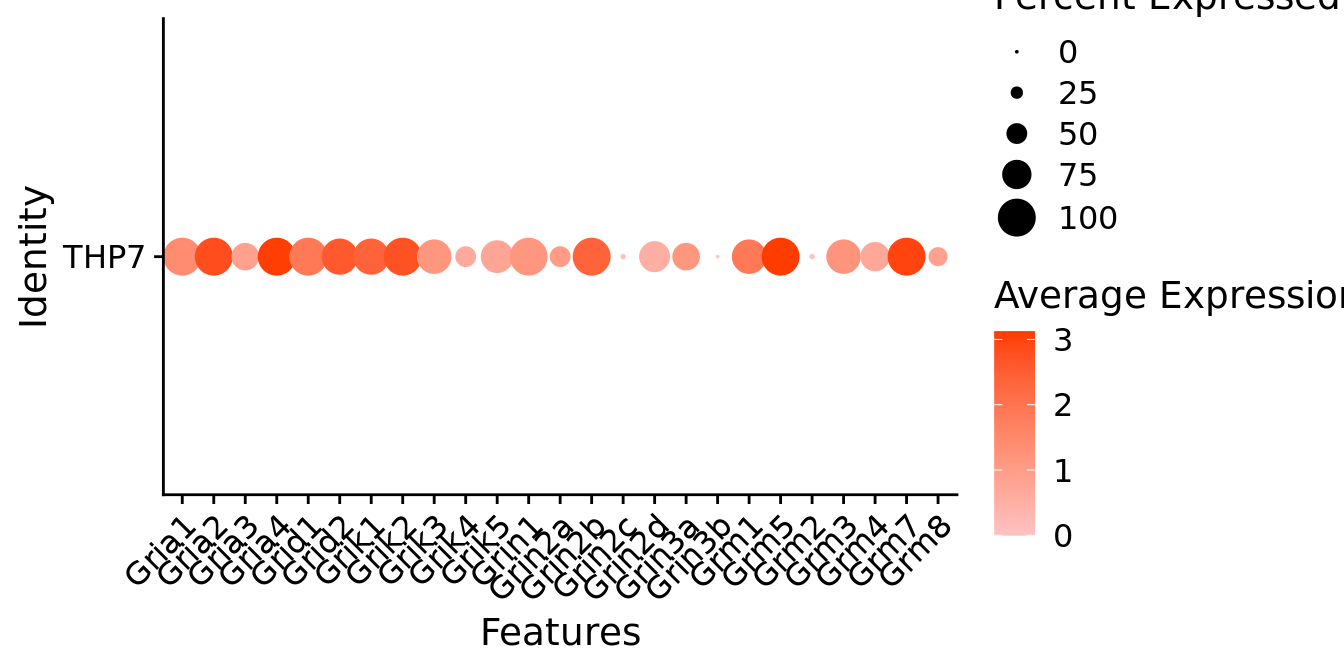
| Version | Author | Date |
|---|---|---|
| 263f215 | Evgenii O. Tretiakov | 2023-03-13 |
DotPlots
Expression of GABA receptors in GABAergic Gal positive cells
DotPlot(object = neuro_fin_neurotrans$GABAergic,
features = gabar,
group.by = "k_tree",
cols = c("#adffff", "#0084ff"),
col.min = -1, col.max = 1
) + RotatedAxis()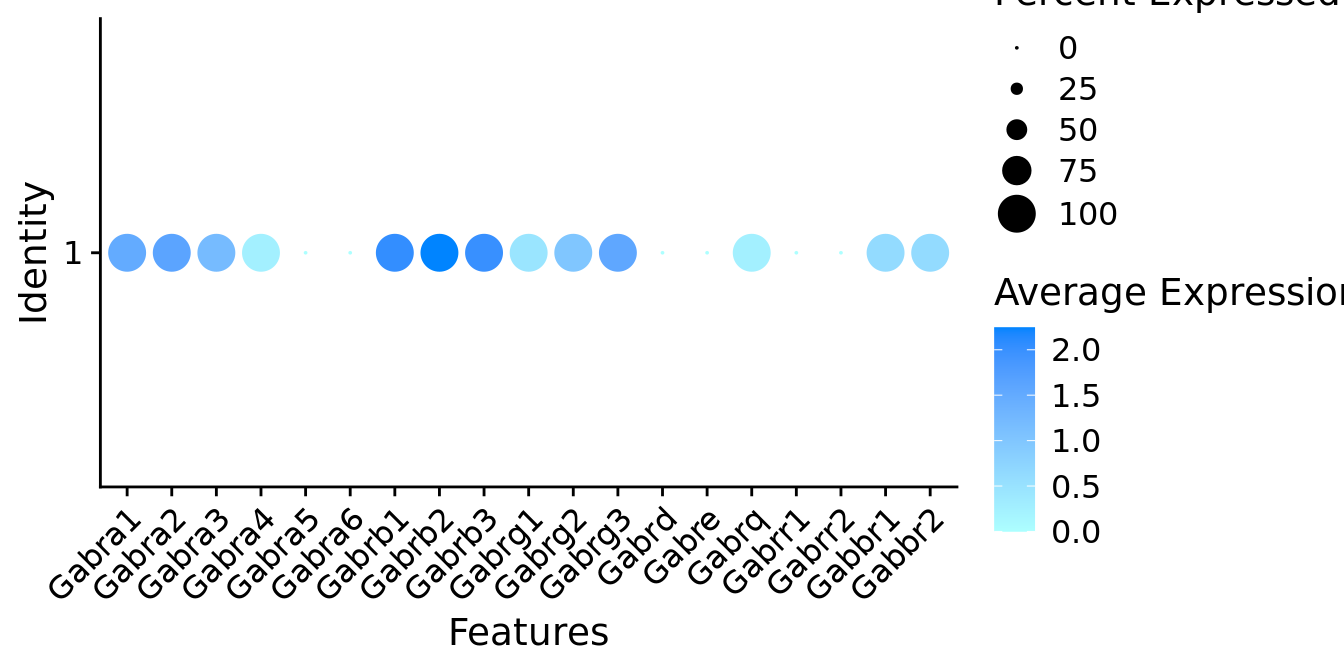
| Version | Author | Date |
|---|---|---|
| 263f215 | Evgenii O. Tretiakov | 2023-03-13 |
Expression of GABA receptors in glutamatergic Gal positive cells
DotPlot(object = neuro_fin_neurotrans$glutamatergic,
features = gabar,
group.by = "k_tree",
cols = c("#ffc2c2", "#ff3c00"),
col.min = -1, col.max = 1
) + RotatedAxis()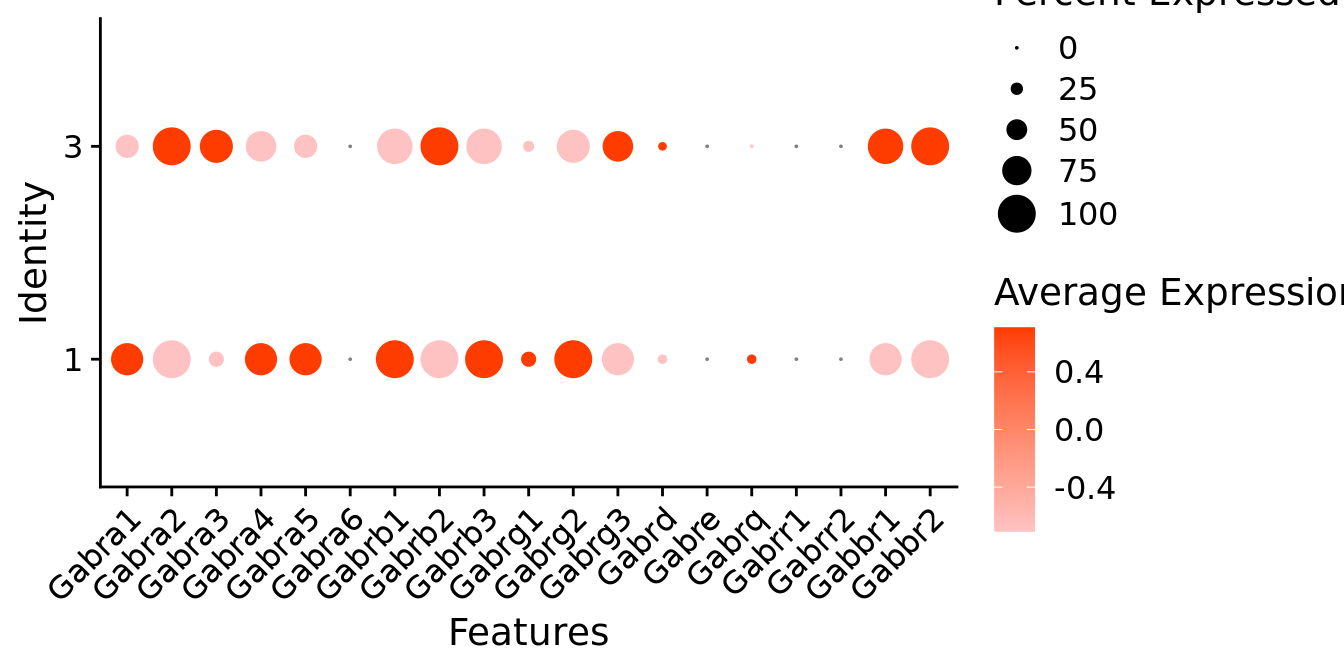
| Version | Author | Date |
|---|---|---|
| 263f215 | Evgenii O. Tretiakov | 2023-03-13 |
Expression of glutamate receptors in GABAergic Gal positive cells
DotPlot(object = neuro_fin_neurotrans$GABAergic,
features = glutr,
group.by = "k_tree",
cols = c("#adffff", "#0084ff"),
col.min = -1, col.max = 1
) + RotatedAxis()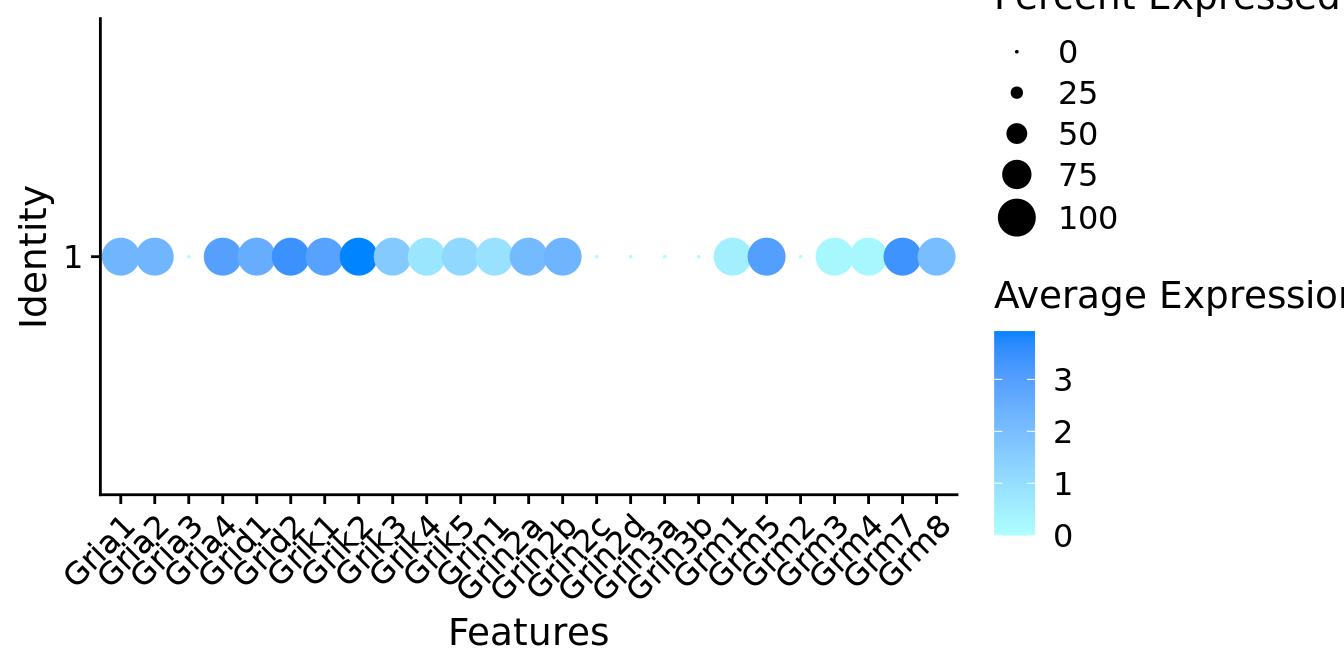
| Version | Author | Date |
|---|---|---|
| 263f215 | Evgenii O. Tretiakov | 2023-03-13 |
Expression of glutamate receptors in glutamatergic Gal positive cells
DotPlot(object = neuro_fin_neurotrans$glutamatergic,
features = glutr,
group.by = "k_tree",
cols = c("#ffc2c2", "#ff3c00"),
col.min = -1, col.max = 1
) + RotatedAxis()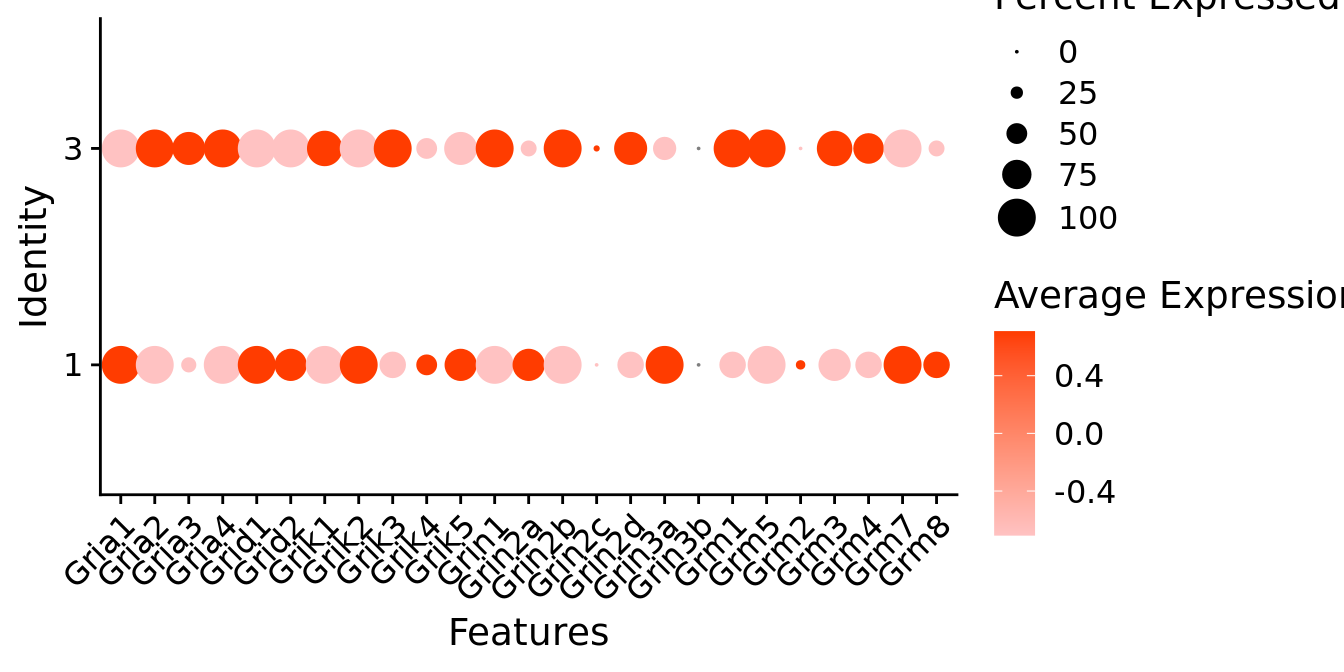
sbs_mtx_neuro <-
neurons %>%
GetAssayData("data", "RNA") %>%
as.data.frame() %>%
t()
rownames(sbs_mtx_neuro) <- colnames(neurons)
# Filter features
filt_low_genes2 <-
colSums(sbs_mtx_neuro) %>%
.[. > quantile(., 0.4)] %>%
names()
sbs_mtx_neuro %<>% .[, filt_low_genes2]
min_filt_vector2 <-
sbs_mtx_neuro %>%
as_tibble() %>%
select(all_of(filt_low_genes2)) %>%
summarise(across(.fns = ~ quantile(.x, .005))) %>%
as.list %>%
map(as.double) %>%
simplify %>%
.[filt_low_genes2]
# Prepare table of intersection sets analysis
content_sbs_mtx_neuro <-
(sbs_mtx_neuro > min_filt_vector2) %>%
as_tibble() %>%
mutate_all(as.numeric)
neurons$gaba_status <-
content_sbs_mtx_neuro %>%
select(Gad1, Gad2, Slc32a1) %>%
mutate(gaba = if_all(.fns = ~ .x > 0)) %>%
.$gaba
neurons$gaba_expr <-
content_sbs_mtx_neuro %>%
select(Gad1, Gad2, Slc32a1) %>%
mutate(gaba = if_any(.fns = ~ .x > 0)) %>%
.$gaba
neurons$glut_status <-
content_sbs_mtx_neuro %>%
select(Slc17a6) %>%
mutate(glut = Slc17a6 > 0) %>%
.$glut
neuro_fin <-
subset(neurons,
cells = union(
WhichCells(neurons,
expression = gaba_status == TRUE & glut_status == FALSE),
WhichCells(neurons,
expression = glut_status == TRUE & gaba_expr == FALSE)))
neuro_fin$status <- neuro_fin$gaba_status %>%
if_else(true = "GABAergic",
false = "glutamatergic")
Idents(neuro_fin) <- "status"
sbs_mtx_neuro <-
neuro_fin %>%
GetAssayData("data", "RNA") %>%
as.data.frame() %>%
t()
rownames(sbs_mtx_neuro) <- colnames(neuro_fin)
# Filter features
filt_low_genes2 <-
colSums(sbs_mtx_neuro) %>%
.[. > quantile(., 0.4)] %>%
names()
sbs_mtx_neuro %<>% .[, filt_low_genes2]
min_filt_vector2 <-
sbs_mtx_neuro %>%
as_tibble() %>%
select(all_of(filt_low_genes2)) %>%
summarise(across(.fns = ~ quantile(.x, .005))) %>%
as.list %>%
map(as.double) %>%
simplify %>%
.[filt_low_genes2]
# Prepare table of intersection sets analysis
content_sbs_mtx_neuro <-
(sbs_mtx_neuro > min_filt_vector2) %>%
as_tibble() %>%
mutate_all(as.numeric)sbs_mtx_neuro_full <- content_sbs_mtx_neuro |>
select(any_of(c(neurotrans, glutr, gabar, "Gal", "Galr1"))) |>
dplyr::bind_cols(neuro_fin@meta.data)
sbs_mtx_neuro_full |> glimpse()Rows: 182
Columns: 102
$ Slc17a6 <dbl> 0, 0, 0, 0, 0, 0, 0, 0, 0, 0, 0, 0, 0, 0…
$ Slc17a7 <dbl> 0, 0, 0, 0, 0, 0, 0, 0, 0, 0, 0, 0, 0, 0…
$ Slc1a1 <dbl> 1, 1, 1, 0, 1, 1, 0, 0, 0, 1, 1, 1, 1, 0…
$ Slc1a2 <dbl> 1, 1, 1, 0, 1, 0, 0, 1, 0, 1, 1, 1, 1, 1…
$ Slc1a6 <dbl> 1, 0, 0, 0, 0, 0, 0, 1, 0, 0, 1, 0, 1, 0…
$ Gad1 <dbl> 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1…
$ Slc32a1 <dbl> 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1…
$ Slc6a1 <dbl> 1, 1, 1, 0, 1, 0, 0, 0, 0, 0, 0, 0, 0, 0…
$ Gria1 <dbl> 0, 1, 1, 1, 0, 1, 1, 1, 0, 1, 0, 0, 1, 1…
$ Gria2 <dbl> 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1…
$ Gria3 <dbl> 1, 1, 1, 1, 1, 1, 0, 1, 1, 1, 1, 1, 1, 1…
$ Gria4 <dbl> 1, 1, 1, 1, 1, 1, 0, 1, 1, 1, 1, 1, 1, 1…
$ Grid1 <dbl> 1, 1, 1, 1, 0, 1, 1, 1, 0, 1, 1, 1, 0, 1…
$ Grid2 <dbl> 1, 1, 1, 1, 1, 0, 0, 0, 1, 1, 0, 0, 0, 1…
$ Grik1 <dbl> 1, 1, 1, 1, 1, 1, 0, 1, 1, 1, 1, 1, 1, 0…
$ Grik2 <dbl> 1, 1, 1, 1, 1, 1, 0, 1, 1, 1, 1, 1, 1, 1…
$ Grik3 <dbl> 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1…
$ Grik4 <dbl> 0, 1, 1, 1, 0, 1, 0, 1, 0, 1, 1, 1, 1, 1…
$ Grik5 <dbl> 1, 1, 1, 1, 1, 1, 0, 1, 1, 1, 1, 1, 1, 1…
$ Grin1 <dbl> 1, 1, 1, 1, 0, 1, 0, 1, 1, 1, 1, 0, 1, 0…
$ Grin2a <dbl> 0, 1, 1, 1, 0, 1, 0, 1, 0, 1, 0, 1, 1, 0…
$ Grin2b <dbl> 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1…
$ Grin2d <dbl> 1, 1, 1, 1, 0, 0, 0, 0, 1, 1, 1, 0, 0, 0…
$ Grin3a <dbl> 1, 1, 1, 1, 1, 1, 0, 1, 1, 1, 1, 1, 1, 1…
$ Grm1 <dbl> 1, 1, 1, 0, 1, 0, 0, 0, 0, 0, 0, 0, 0, 1…
$ Grm5 <dbl> 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1…
$ Grm2 <dbl> 0, 1, 0, 0, 0, 0, 0, 0, 0, 0, 0, 0, 0, 0…
$ Grm3 <dbl> 1, 1, 1, 1, 1, 1, 0, 1, 1, 1, 1, 1, 1, 1…
$ Grm4 <dbl> 0, 1, 0, 0, 0, 0, 1, 0, 1, 0, 0, 0, 0, 0…
$ Grm7 <dbl> 1, 1, 1, 1, 1, 1, 1, 1, 0, 1, 1, 1, 1, 1…
$ Grm8 <dbl> 1, 1, 1, 1, 1, 1, 1, 1, 0, 1, 1, 0, 1, 0…
$ Gabra1 <dbl> 0, 1, 0, 0, 0, 0, 0, 0, 0, 0, 0, 0, 0, 0…
$ Gabra2 <dbl> 1, 1, 1, 1, 1, 0, 1, 1, 0, 1, 0, 0, 0, 1…
$ Gabra3 <dbl> 1, 1, 1, 1, 1, 1, 0, 1, 0, 1, 1, 0, 1, 1…
$ Gabra4 <dbl> 1, 1, 0, 0, 0, 0, 1, 1, 1, 1, 0, 0, 0, 0…
$ Gabra5 <dbl> 1, 1, 1, 1, 0, 0, 0, 1, 0, 0, 0, 0, 1, 0…
$ Gabrb1 <dbl> 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1…
$ Gabrb2 <dbl> 1, 1, 1, 1, 1, 1, 1, 0, 0, 0, 0, 0, 0, 1…
$ Gabrb3 <dbl> 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1…
$ Gabrg1 <dbl> 1, 0, 0, 0, 0, 0, 0, 0, 1, 0, 0, 0, 0, 1…
$ Gabrg2 <dbl> 1, 0, 0, 1, 1, 1, 0, 0, 0, 1, 0, 0, 1, 0…
$ Gabrg3 <dbl> 1, 1, 1, 1, 1, 1, 0, 1, 1, 1, 1, 1, 1, 1…
$ Gabrd <dbl> 0, 0, 0, 0, 0, 0, 0, 0, 0, 0, 0, 0, 0, 0…
$ Gabbr1 <dbl> 1, 1, 1, 1, 0, 1, 0, 1, 1, 1, 1, 1, 1, 1…
$ Gabbr2 <dbl> 1, 1, 1, 1, 1, 1, 0, 1, 1, 1, 1, 1, 1, 1…
$ Gal <dbl> 0, 0, 0, 0, 0, 0, 0, 0, 0, 0, 0, 0, 0, 0…
$ nCount_RAW <dbl> 37619, 44705, 26662, 19861, 7835, 30715,…
$ nFeature_RAW <int> 7847, 8422, 7117, 5535, 3264, 6150, 1849…
$ nCount_SCT <dbl> 6740, 6680, 6723, 6418, 7213, 6791, 5390…
$ nFeature_SCT <int> 3279, 3367, 3466, 2643, 3237, 2698, 1964…
$ nCount_RNA <dbl> 37384, 44410, 26513, 19730, 7793, 30488,…
$ nFeature_RNA <int> 7842, 8397, 7110, 5524, 3256, 6132, 1843…
$ log10GenesPerUMI <dbl> 0.8516717, 0.8443551, 0.8707823, 0.87127…
$ percent_mito <dbl> 0.018724588, 0.006755235, 0.000000000, 0…
$ percent_ribo <dbl> 0.3450674, 0.1959018, 0.3356844, 0.20273…
$ percent_hb <dbl> 0.016049647, 0.013510471, 0.007543469, 0…
$ var_regex <dbl> 3.635245, 3.341590, 4.692038, 3.938165, …
$ S.Score <dbl> -0.0117061756, 0.0006385497, -0.05347786…
$ G2M.Score <dbl> -0.039302759, -0.085392871, -0.049283519…
$ log_prob_doublet <dbl> -1992.42534, -2481.72723, -1981.02186, -…
$ orig.ident <chr> "THP7", "THP7", "THP7", "THP7", "THP7", …
$ comb_clstr1 <fct> 10, 10, 10, 10, 10, 10, 7, 10, 10, 10, 1…
$ comb_clstr2 <fct> 8, 8, 8, 8, 8, 8, 10, 8, 8, 8, 8, 8, 8, …
$ QC <chr> "Pass", "Pass", "Pass", "Pass", "Pass", …
$ cell_name <chr> "THP7_ACTTTCAAGGATTTGA-1", "THP7_CATCCCA…
$ RNA_snn_res.0.2 <fct> 4, 1, 4, 4, 4, 4, 1, 4, 4, 4, 4, 4, 4, 3…
$ RNA_snn_res.0.226763010217232 <fct> 5, 1, 5, 5, 5, 5, 1, 5, 5, 5, 5, 5, 5, 3…
$ RNA_snn_res.0.254822164960175 <fct> 6, 1, 6, 6, 6, 6, 1, 6, 6, 6, 6, 6, 6, 4…
$ RNA_snn_res.0.284273960186496 <fct> 6, 1, 6, 6, 6, 6, 1, 6, 6, 6, 6, 6, 6, 4…
$ RNA_snn_res.0.315224714241019 <fct> 6, 1, 6, 6, 6, 6, 1, 6, 6, 6, 6, 6, 6, 4…
$ RNA_snn_res.0.347791850274373 <fct> 6, 1, 6, 6, 6, 6, 1, 6, 6, 6, 6, 6, 6, 4…
$ RNA_snn_res.0.382105384969072 <fct> 6, 1, 6, 6, 6, 6, 1, 6, 6, 6, 6, 6, 6, 4…
$ RNA_snn_res.0.41830966336087 <fct> 6, 1, 6, 6, 6, 6, 1, 6, 6, 6, 6, 6, 6, 4…
$ RNA_snn_res.0.4565653885612 <fct> 6, 1, 6, 6, 6, 6, 1, 6, 6, 6, 6, 6, 6, 4…
$ RNA_snn_res.0.49705200657103 <fct> 6, 1, 6, 6, 6, 6, 1, 6, 6, 6, 6, 6, 6, 4…
$ RNA_snn_res.0.539970520838189 <fct> 6, 1, 6, 6, 6, 6, 1, 6, 6, 6, 6, 6, 6, 4…
$ RNA_snn_res.0.585546829701983 <fct> 6, 1, 6, 6, 6, 6, 1, 6, 6, 6, 6, 6, 6, 4…
$ RNA_snn_res.0.634035703678448 <fct> 6, 1, 6, 6, 6, 6, 1, 6, 6, 6, 6, 6, 6, 4…
$ RNA_snn_res.0.685725550422073 <fct> 6, 1, 6, 6, 6, 6, 1, 6, 6, 6, 6, 6, 6, 4…
$ RNA_snn_res.0.740944155569623 <fct> 5, 3, 5, 5, 5, 5, 3, 5, 5, 5, 5, 5, 5, 4…
$ RNA_snn_res.0.800065640881591 <fct> 5, 3, 5, 5, 5, 5, 3, 5, 5, 5, 5, 5, 5, 4…
$ RNA_snn_res.0.863518951850731 <fct> 5, 3, 5, 5, 5, 5, 3, 5, 5, 5, 5, 5, 5, 4…
$ RNA_snn_res.0.931798281916928 <fct> 5, 7, 5, 5, 5, 5, 3, 5, 5, 5, 5, 5, 5, 2…
$ RNA_snn_res.1.00547596918591 <fct> 5, 7, 5, 5, 5, 5, 3, 5, 5, 5, 5, 5, 5, 2…
$ RNA_snn_res.1.08521857800307 <fct> 5, 7, 5, 5, 5, 5, 3, 5, 5, 5, 5, 5, 5, 2…
$ RNA_snn_res.1.17180712235645 <fct> 5, 7, 5, 5, 5, 5, 3, 5, 5, 5, 5, 5, 5, 2…
$ RNA_snn_res.1.26616273144792 <fct> 5, 6, 5, 5, 5, 5, 3, 5, 5, 5, 5, 5, 5, 2…
$ RNA_snn_res.1.36937954624667 <fct> 5, 6, 5, 5, 5, 5, 3, 5, 5, 5, 5, 5, 5, 2…
$ RNA_snn_res.1.48278948455203 <fct> 4, 5, 4, 4, 4, 4, 10, 4, 4, 4, 4, 4, 4, …
$ RNA_snn_res.1.60797961788652 <fct> 4, 5, 4, 4, 4, 4, 10, 4, 4, 4, 4, 4, 4, …
$ RNA_snn_res.1.74695704064407 <fct> 4, 5, 4, 4, 4, 4, 10, 4, 4, 4, 4, 4, 4, …
$ RNA_snn_res.1.90211850288799 <fct> 4, 5, 4, 4, 4, 4, 10, 4, 4, 4, 4, 4, 4, …
$ RNA_snn_res.2.07648919562638 <fct> 3, 5, 3, 3, 3, 3, 10, 3, 3, 3, 3, 3, 3, …
$ RNA_snn_res.2.27407915710999 <fct> 3, 5, 3, 3, 3, 3, 10, 3, 3, 3, 3, 3, 3, …
$ RNA_snn_res.2.50001 <fct> 4, 5, 4, 4, 4, 4, 11, 4, 4, 4, 4, 4, 4, …
$ seurat_clusters <fct> 4, 5, 4, 4, 4, 4, 11, 4, 4, 4, 4, 4, 4, …
$ k_tree <fct> 6, 1, 6, 6, 6, 6, 1, 6, 6, 6, 6, 6, 6, 4…
$ sep_clstr <fct> 6, 1, 6, 6, 6, 6, 1, 6, 6, 6, 6, 6, 6, 4…
$ gaba_status <lgl> TRUE, TRUE, TRUE, TRUE, TRUE, TRUE, TRUE…
$ gaba_expr <lgl> TRUE, TRUE, TRUE, TRUE, TRUE, TRUE, TRUE…
$ glut_status <lgl> FALSE, FALSE, FALSE, FALSE, FALSE, FALSE…
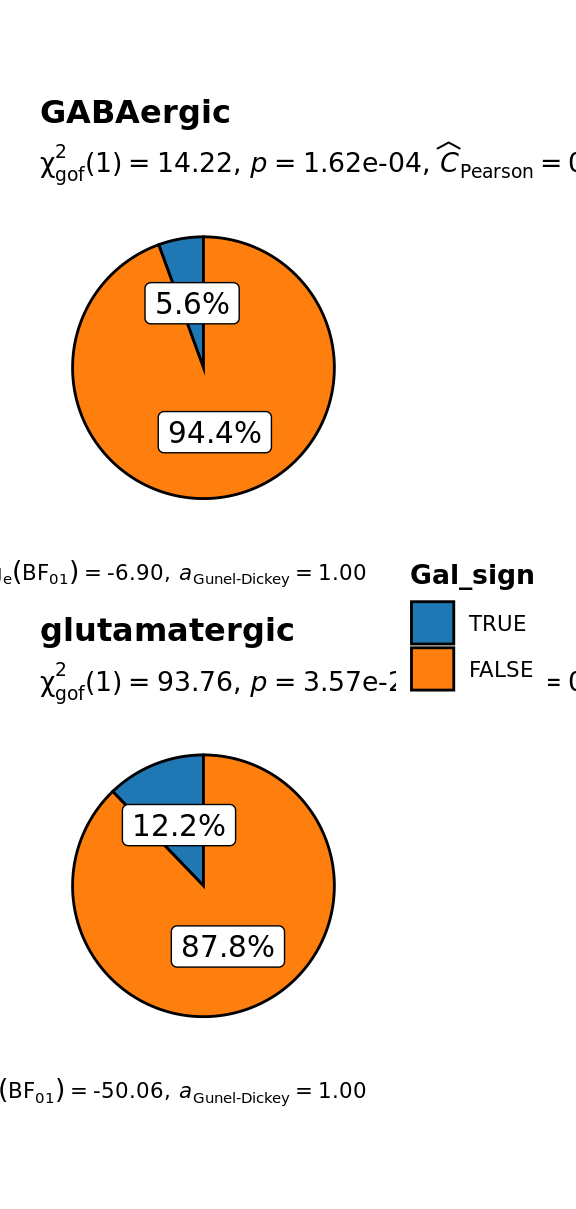
$ status <chr> "GABAergic", "GABAergic", "GABAergic", "…sbs_mtx_neuro_full$Gal_sign <-
sbs_mtx_neuro_full %>%
select(Gal) %>%
mutate(Gal_sign = if_any(.fns = ~ .x > 0)) %>%
.$Gal_sign
# for reproducibility
set.seed(reseed)
# plot
grouped_ggpiestats(
# arguments relevant for `ggpiestats()`
data = sbs_mtx_neuro_full,
x = Gal_sign,
grouping.var = status,
perc.k = 1,
package = "ggsci",
palette = "category10_d3",
# arguments relevant for `combine_plots()`
title.text = "Molecular specification of thalamic neuronal lineages by Gal signalling and main neurotransmitter expression",
caption.text = "Asterisks denote results from proportion tests; \n***: p < 0.001, ns: non-significant",
plotgrid.args = list(nrow = 2)
)
| Version | Author | Date |
|---|---|---|
| 263f215 | Evgenii O. Tretiakov | 2023-03-13 |
p1 <- FeaturePlot_scCustom(
srt, "Slc17a6",
pt.size = 2,
order = TRUE,
alpha_exp = 0.65,
alpha_na_exp = 0.2,
label = TRUE,
repel = TRUE,
colors_use = srt@misc$expr_Colour_Pal) +
ggtitle("Slc17a6(Vglut2): ") + theme(plot.title = element_text(size = 24))
p1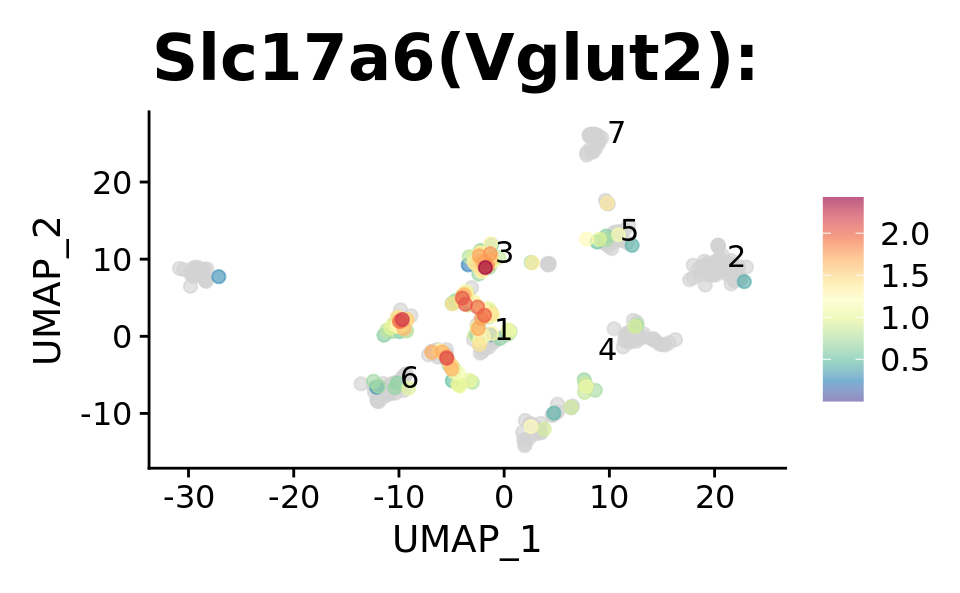
Plot_Density_Custom(
seurat_object = srt,
features = "Slc17a6",
pt.size = 2) +
ggtitle("Slc17a6(Vglut2): ") + theme(plot.title = element_text(size = 24))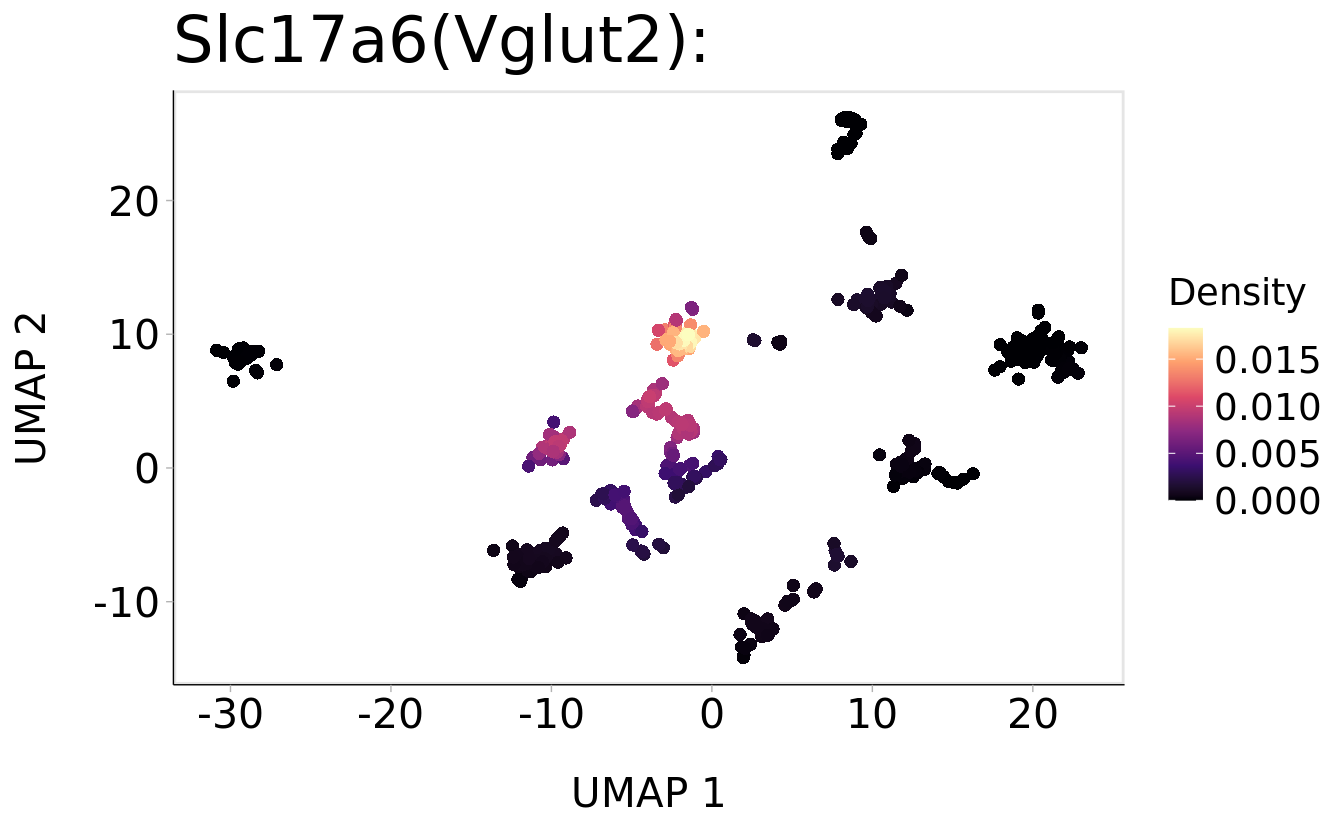
| Version | Author | Date |
|---|---|---|
| 4ef209f | Evgenii O. Tretiakov | 2023-03-05 |
Plot_Density_Joint_Only(
seurat_object = srt,
features = c("Slc17a6", "Gal"),
pt.size = 2) +
ggtitle("Slc17a6(Vglut2) + Gal ") + theme(plot.title = element_text(size = 24))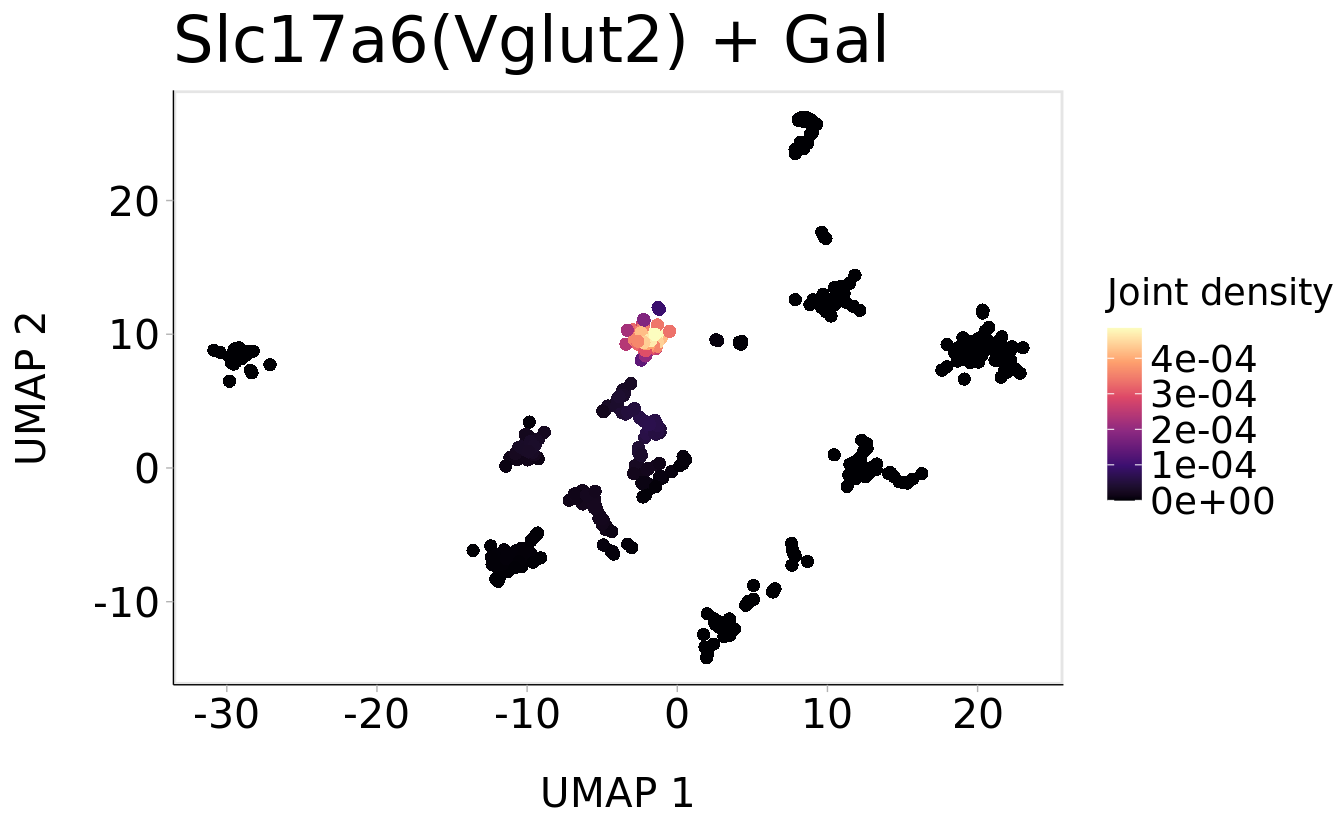
| Version | Author | Date |
|---|---|---|
| 4ef209f | Evgenii O. Tretiakov | 2023-03-05 |
srt_vglut2 <- subset(srt, idents = c(2, 4, 5, 6, 7), invert = TRUE)
srt_vglut2 <- subset(srt_vglut2, subset = UMAP_1 > -15 & UMAP_1 < 2)p2 <- FeaturePlot_scCustom(
srt_vglut2, "Gal",
pt.size = 5,
order = TRUE,
alpha_exp = 0.75,
alpha_na_exp = 0.05,
colors_use = srt@misc$expr_Colour_Pal) +
ggtitle("Gal in Vglut2+ populations") + theme(plot.title = element_text(size = 24))
p2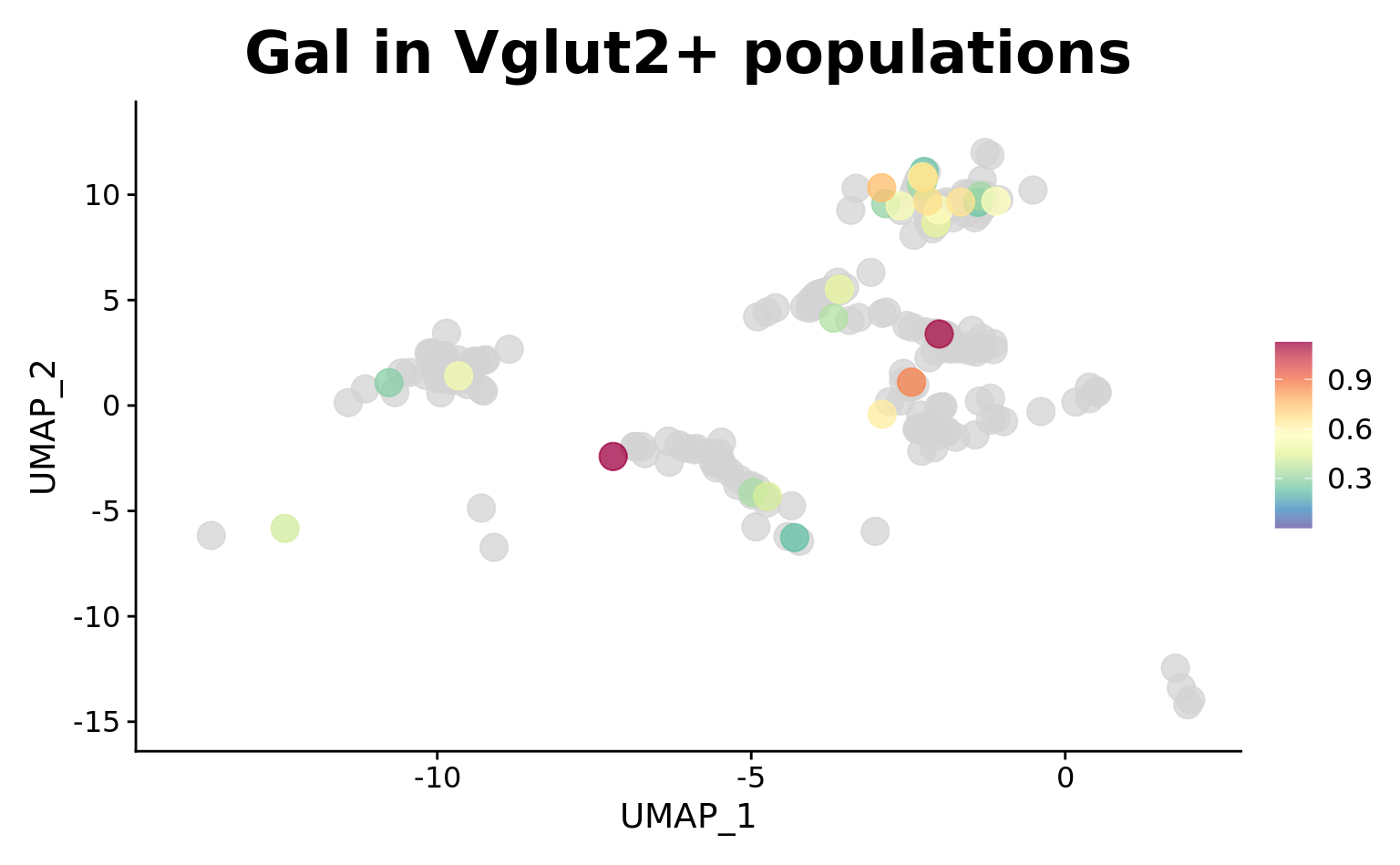
| Version | Author | Date |
|---|---|---|
| 4ef209f | Evgenii O. Tretiakov | 2023-03-05 |
Plot_Density_Custom(
seurat_object = srt_vglut2,
features = "Gal",
pt.size = 2) +
ggtitle("Gal across Vglut cell-populations only") + theme(plot.title = element_text(size = 24))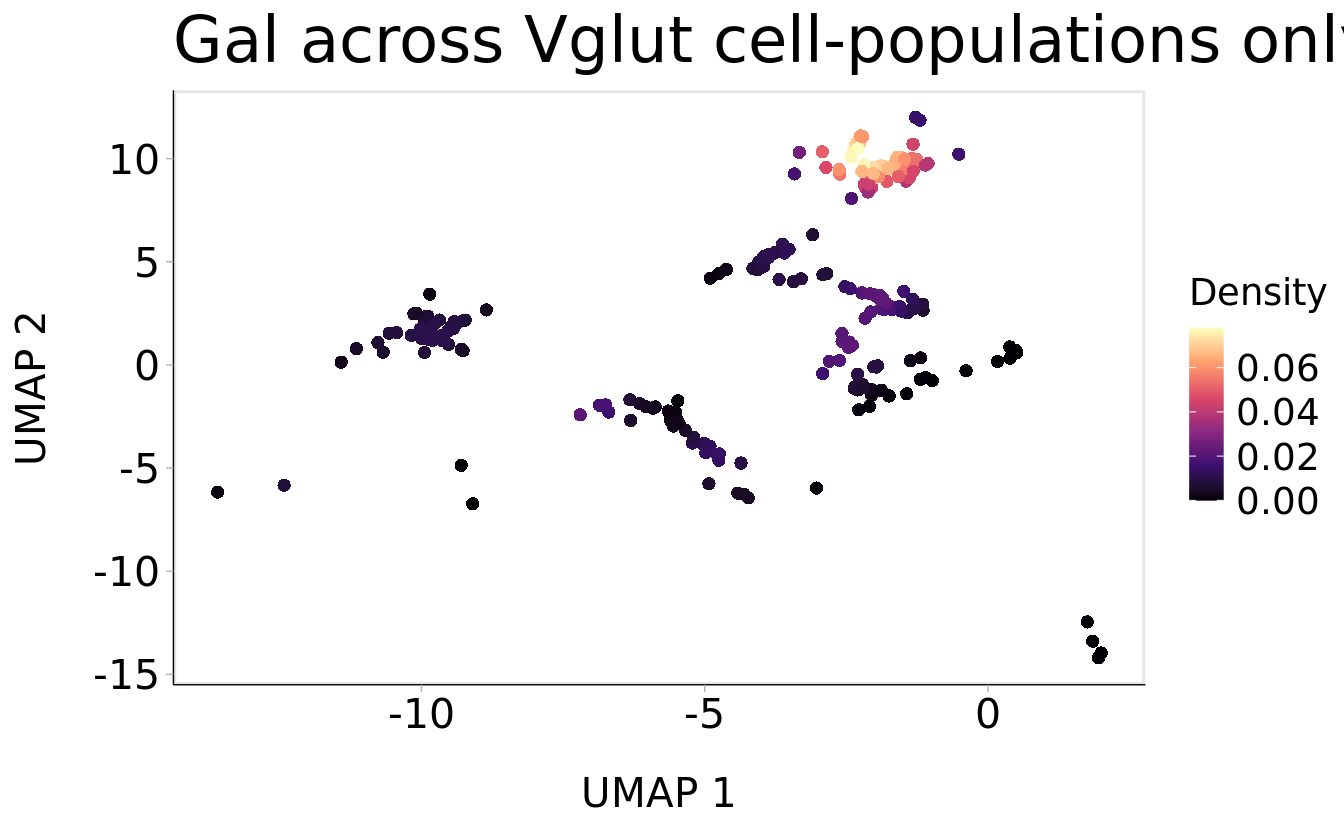
| Version | Author | Date |
|---|---|---|
| 4ef209f | Evgenii O. Tretiakov | 2023-03-05 |
Plot_Density_Joint_Only(
seurat_object = srt_vglut2,
features = c("Slc17a6", "Gal"),
pt.size = 2) +
ggtitle("Slc17a6 + Gal across Vglut cell-populations only") + theme(plot.title = element_text(size = 24))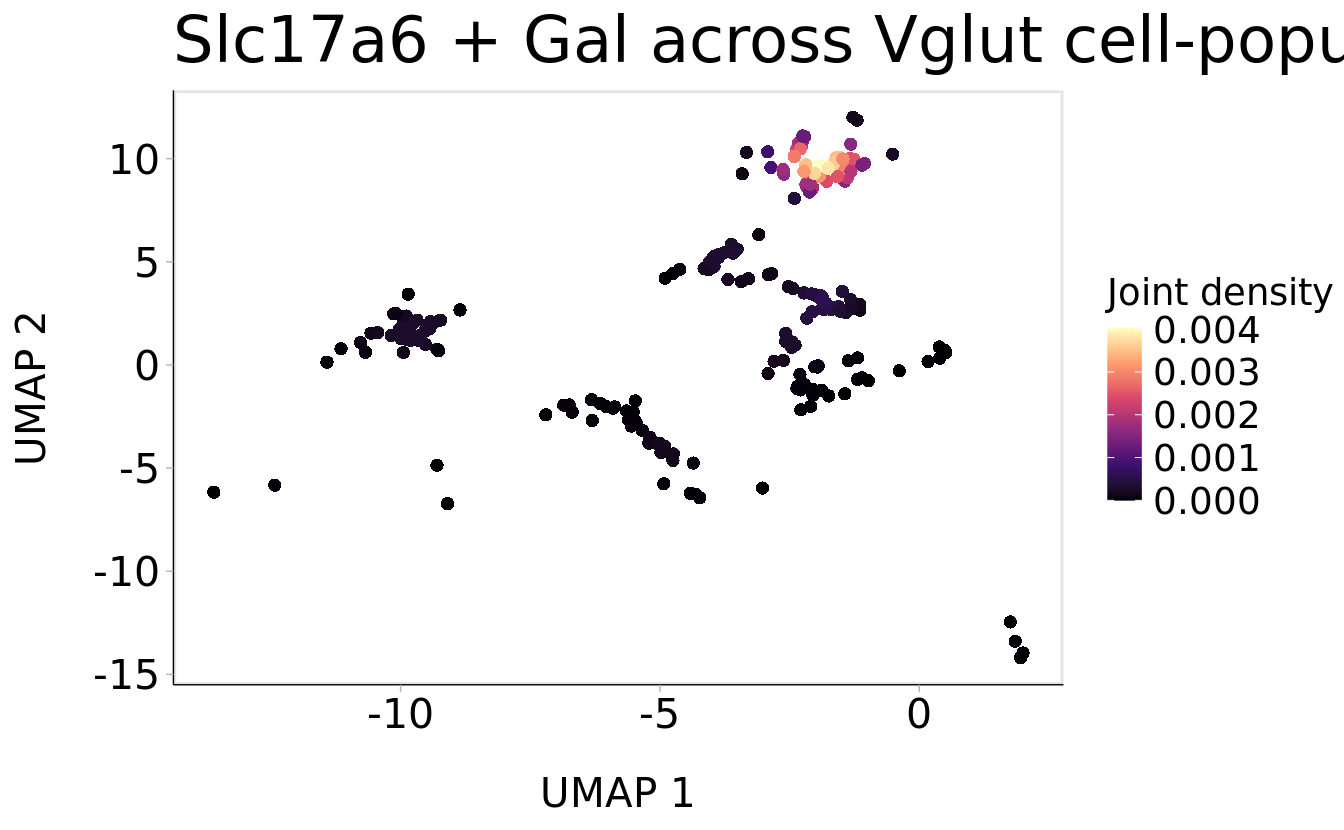
| Version | Author | Date |
|---|---|---|
| 4ef209f | Evgenii O. Tretiakov | 2023-03-05 |
DimPlot_scCustom(
srt_vglut2,
label = TRUE,
repel = TRUE,
pt.size = 3,
figure_plot = TRUE
) + ggtitle("Vglut2+ clusters") + NoLegend()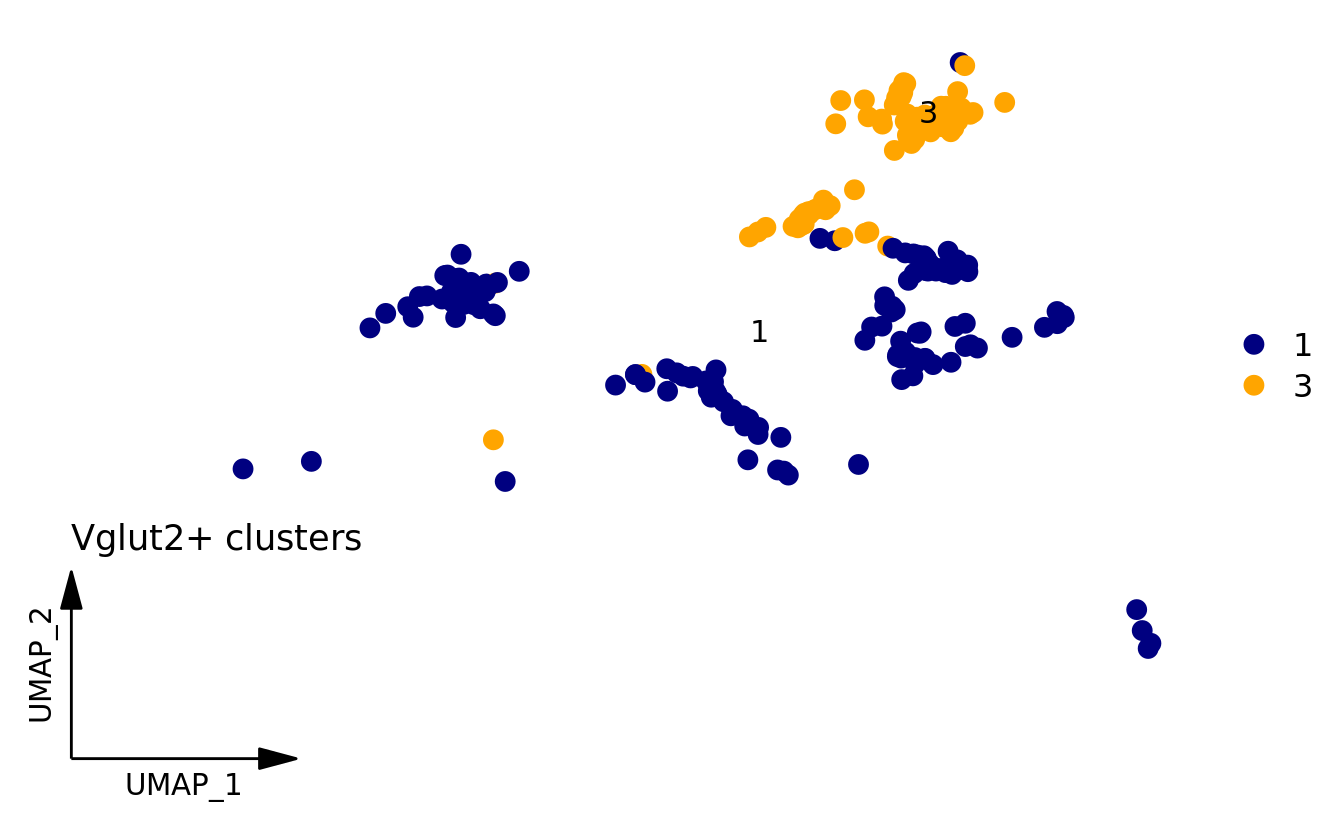
| Version | Author | Date |
|---|---|---|
| 4ef209f | Evgenii O. Tretiakov | 2023-03-05 |
DoHeatmap(srt_vglut2, features = genes_present$found_features)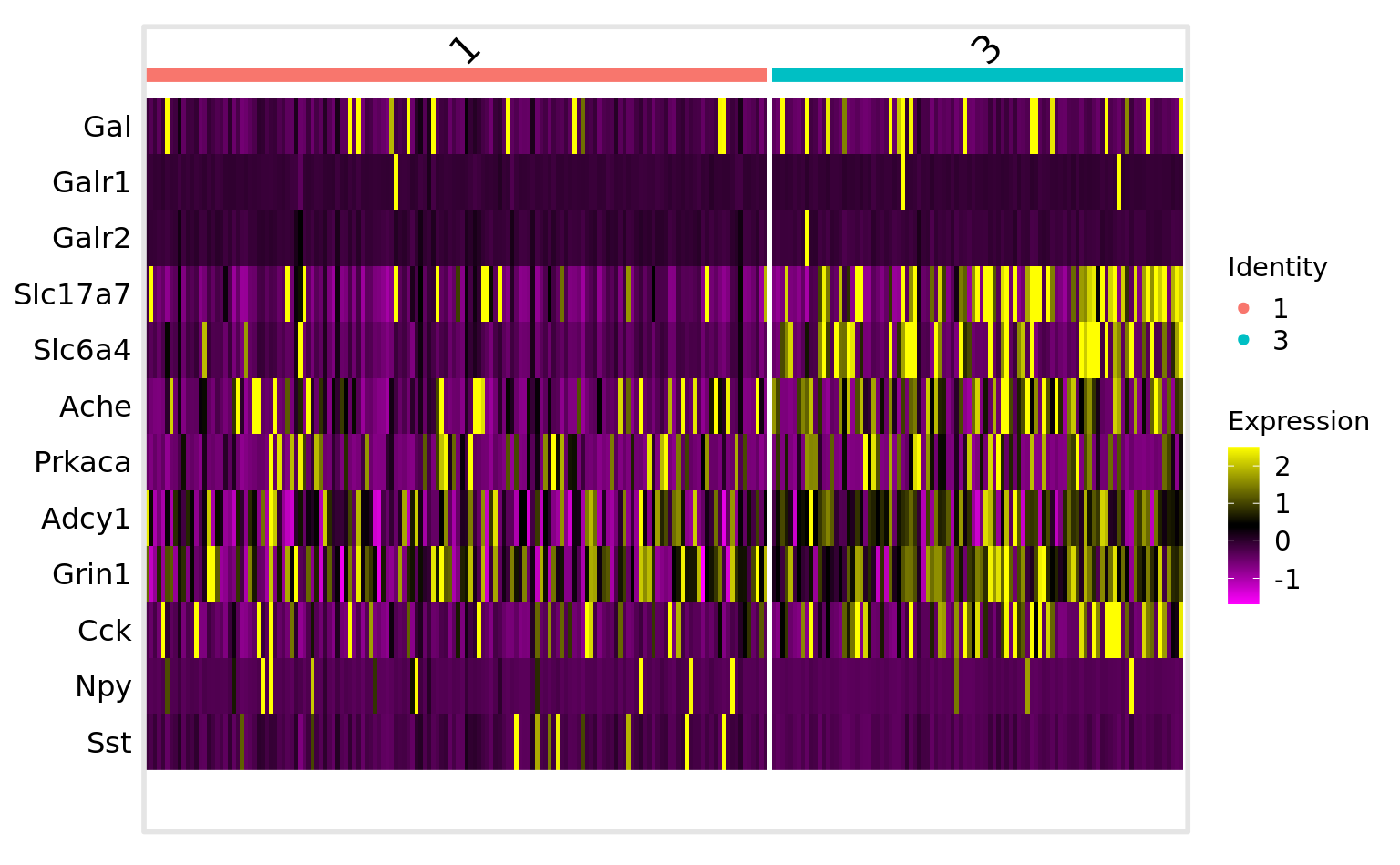
p3 <- DotPlot_scCustom(
seurat_object = srt_vglut2,
assay = "RNA",
features = genes_present$found_features,
flip_axes = TRUE,
x_lab_rotate = TRUE,
colors_use = viridis(n = 30, alpha = .75, direction = -1, option = "E"))
p3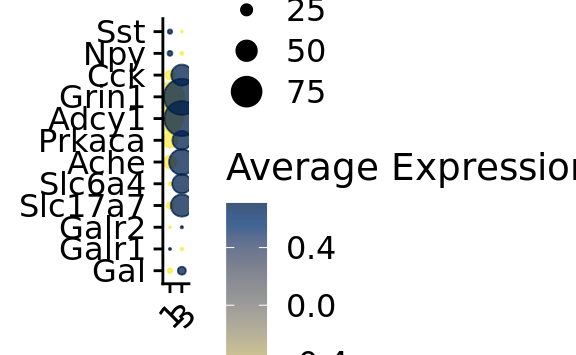
p4 <- Stacked_VlnPlot(
seurat_object = srt_vglut2,
assay = "RNA",
features = genes_present$found_features,
x_lab_rotate = F
)
p4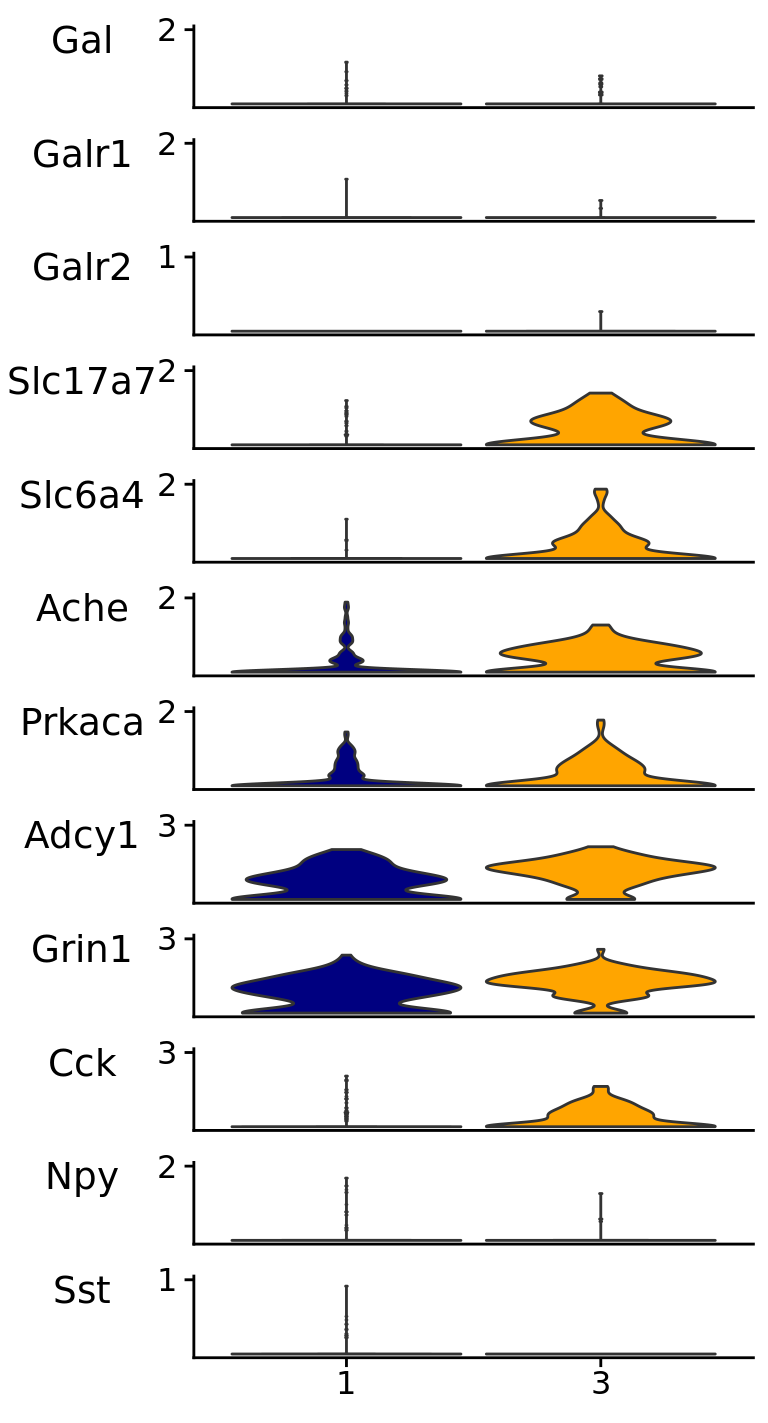
| Version | Author | Date |
|---|---|---|
| 9014631 | Evgenii O. Tretiakov | 2023-08-17 |
p1 | p2 | p3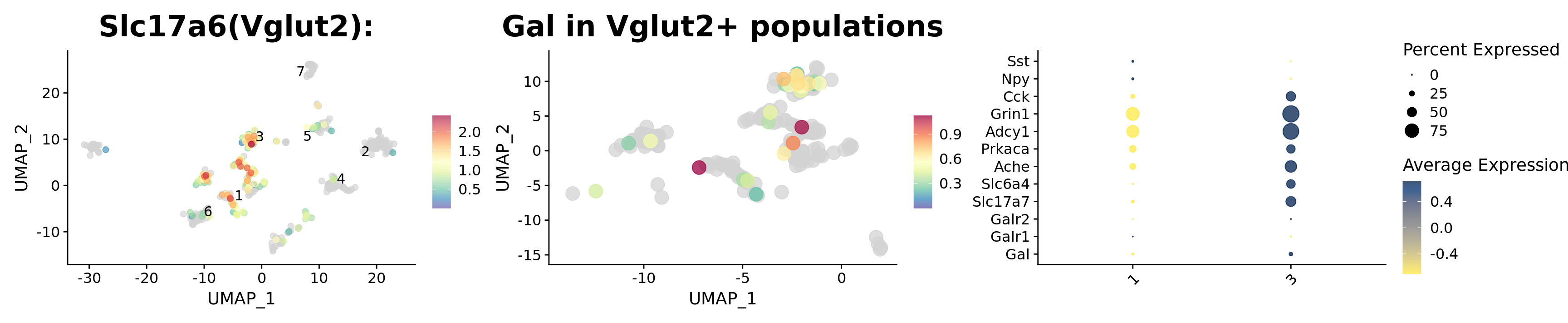
(p1 / p2) | p4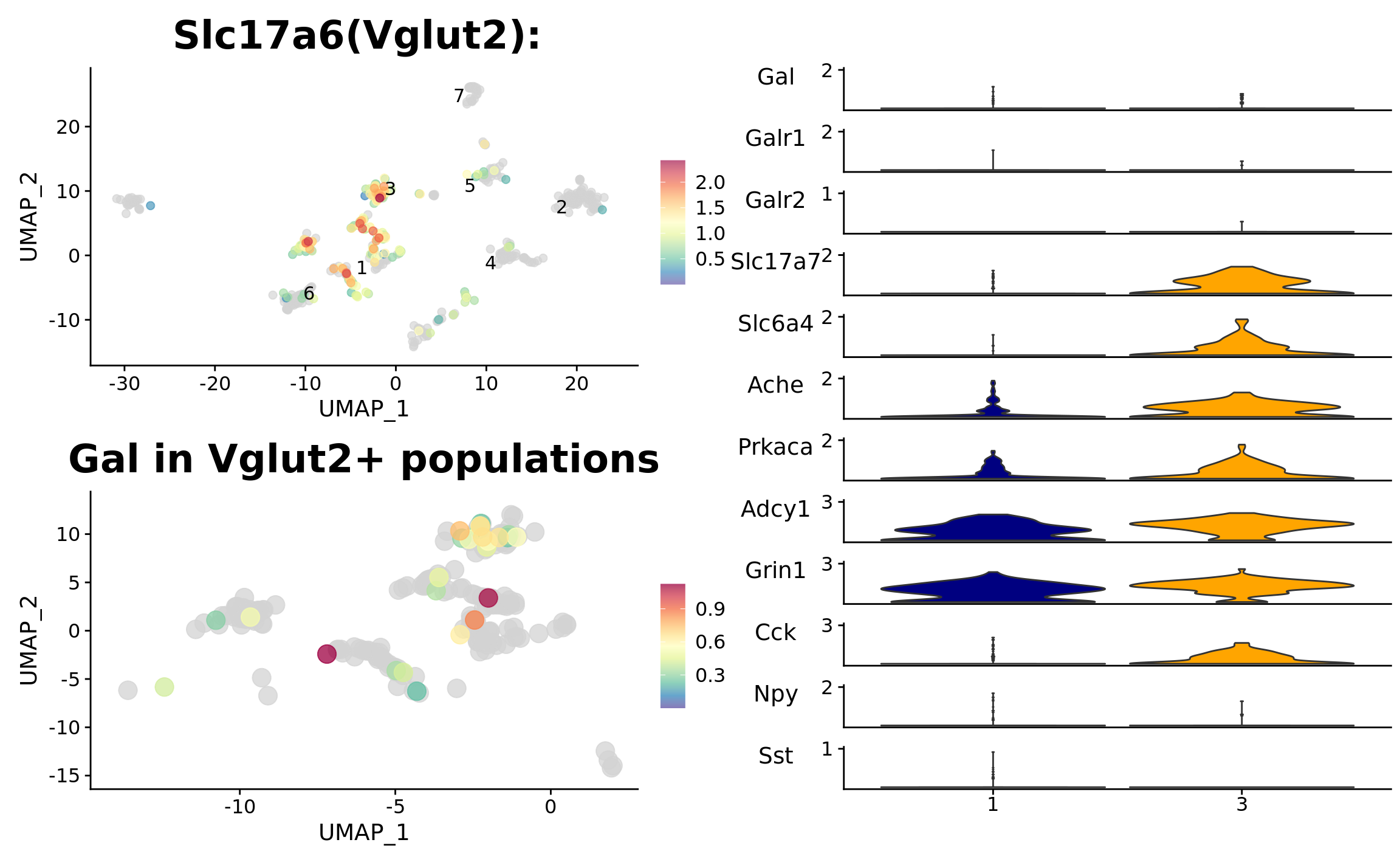
| Version | Author | Date |
|---|---|---|
| 9014631 | Evgenii O. Tretiakov | 2023-08-17 |
sessionInfo()R version 4.3.1 (2023-06-16)
Platform: x86_64-pc-linux-gnu (64-bit)
Running under: Ubuntu 22.04.3 LTS
Matrix products: default
BLAS: /usr/lib/x86_64-linux-gnu/openblas-pthread/libblas.so.3
LAPACK: /usr/lib/x86_64-linux-gnu/openblas-pthread/libopenblasp-r0.3.20.so; LAPACK version 3.10.0
locale:
[1] LC_CTYPE=en_US.UTF-8 LC_NUMERIC=C
[3] LC_TIME=en_US.UTF-8 LC_COLLATE=en_US.UTF-8
[5] LC_MONETARY=en_US.UTF-8 LC_MESSAGES=en_US.UTF-8
[7] LC_PAPER=en_US.UTF-8 LC_NAME=C
[9] LC_ADDRESS=C LC_TELEPHONE=C
[11] LC_MEASUREMENT=en_US.UTF-8 LC_IDENTIFICATION=C
time zone: Etc/UTC
tzcode source: system (glibc)
attached base packages:
[1] stats graphics grDevices utils datasets methods base
other attached packages:
[1] gprofiler2_0.2.2 mrtree_0.0.0.9000 Nebulosa_1.10.0
[4] scCustomize_1.1.3 Scillus_0.5.0 qs_0.25.5
[7] ggstatsplot_0.12.0.9000 UpSetR_1.4.0 patchwork_1.1.3.9000
[10] glmGamPoi_1.12.2 sctransform_0.3.5 SeuratDisk_0.0.0.9020
[13] SeuratWrappers_0.3.1 SeuratObject_4.1.3 Seurat_4.3.0
[16] reticulate_1.31 kableExtra_1.3.4 zeallot_0.1.0
[19] future_1.33.0 skimr_2.1.5 magrittr_2.0.3
[22] lubridate_1.9.2 forcats_1.0.0 stringr_1.5.0
[25] dplyr_1.1.2 purrr_1.0.2 readr_2.1.4
[28] tidyr_1.3.0 tibble_3.2.1 ggplot2_3.4.3
[31] tidyverse_2.0.0.9000 viridis_0.6.4 viridisLite_0.4.2
[34] RColorBrewer_1.1-3 here_1.0.1 workflowr_1.7.0
loaded via a namespace (and not attached):
[1] IRanges_2.34.1 R.methodsS3_1.8.2
[3] goftest_1.2-3 phytools_1.9-16
[5] TH.data_1.1-2 vctrs_0.6.3
[7] spatstat.random_3.1-5 RApiSerialize_0.1.2
[9] effectsize_0.8.5 digest_0.6.33
[11] png_0.1-8 shape_1.4.6
[13] git2r_0.32.0 ggrepel_0.9.3
[15] bayestestR_0.13.1 correlation_0.8.4
[17] deldir_1.0-9 parallelly_1.36.0
[19] combinat_0.0-8 magick_2.7.5
[21] MASS_7.3-60 reshape2_1.4.4
[23] httpuv_1.6.11 foreach_1.5.2
[25] BiocGenerics_0.46.0 withr_2.5.0
[27] ggrastr_1.0.2 xfun_0.40
[29] ggfun_0.1.2 ellipsis_0.3.2
[31] survival_3.5-7 memoise_2.0.1
[33] ggbeeswarm_0.7.2 clustree_0.5.0
[35] MatrixModels_0.5-2 janitor_2.2.0.9000
[37] emmeans_1.8.7 parameters_0.21.1
[39] systemfonts_1.0.4 tidytree_0.4.5
[41] zoo_1.8-12 GlobalOptions_0.1.2
[43] pbapply_1.7-2 R.oo_1.25.0
[45] datawizard_0.8.0 rematch2_2.1.2
[47] promises_1.2.1 scatterplot3d_0.3-44
[49] httr_1.4.7 globals_0.16.2
[51] fitdistrplus_1.1-11 ps_1.7.5
[53] stringfish_0.15.8 rstudioapi_0.15.0
[55] miniUI_0.1.1.1 generics_0.1.3
[57] base64enc_0.1-3 processx_3.8.2
[59] S4Vectors_0.38.1 repr_1.1.6
[61] zlibbioc_1.46.0 ggraph_2.1.0.9000
[63] polyclip_1.10-4 quadprog_1.5-8
[65] GenomeInfoDbData_1.2.10 xtable_1.8-4
[67] pracma_2.4.2 doParallel_1.0.17
[69] evaluate_0.21 S4Arrays_1.0.5
[71] hms_1.1.3 GenomicRanges_1.52.0
[73] irlba_2.3.5.1 colorspace_2.1-0
[75] hdf5r_1.3.8 ROCR_1.0-11
[77] spatstat.data_3.0-1 lmtest_0.9-40
[79] snakecase_0.11.0 ggtree_3.9.1
[81] later_1.3.1 lattice_0.21-8
[83] spatstat.geom_3.2-4 future.apply_1.11.0
[85] getPass_0.2-2 scattermore_1.2
[87] cowplot_1.1.1 matrixStats_1.0.0
[89] RcppAnnoy_0.0.21 pillar_1.9.0
[91] nlme_3.1-163 iterators_1.0.14
[93] compiler_4.3.1 RSpectra_0.16-1
[95] stringi_1.7.12 dendextend_1.17.1
[97] tensor_1.5 SummarizedExperiment_1.30.2
[99] plyr_1.8.8 crayon_1.5.2
[101] abind_1.4-5 gridGraphics_0.5-1
[103] sp_2.0-0 graphlayouts_1.0.0
[105] bit_4.0.5 sandwich_3.0-2
[107] fastmatch_1.1-3 whisker_0.4.1
[109] codetools_0.2-19 multcomp_1.4-25
[111] bslib_0.5.1 paletteer_1.5.0
[113] GetoptLong_1.0.5 plotly_4.10.2
[115] mime_0.12 splines_4.3.1
[117] circlize_0.4.16 Rcpp_1.0.11
[119] prismatic_1.1.1 knitr_1.43
[121] utf8_1.2.3 clue_0.3-64
[123] fs_1.6.3 listenv_0.9.0
[125] checkmate_2.2.0 expm_0.999-7
[127] ggplotify_0.1.2 estimability_1.4.1
[129] Matrix_1.6-1 callr_3.7.3
[131] tzdb_0.4.0 svglite_2.1.1
[133] tweenr_2.0.2 pkgconfig_2.0.3
[135] tools_4.3.1 cachem_1.0.8
[137] numDeriv_2016.8-1.1 rvest_1.0.3
[139] fastmap_1.1.1 rmarkdown_2.24
[141] scales_1.2.1 grid_4.3.1
[143] ica_1.0-3 sass_0.4.7
[145] coda_0.19-4 ggprism_1.0.4
[147] BiocManager_1.30.22 insight_0.19.3.2
[149] RANN_2.6.1 ggimage_0.3.3
[151] farver_2.1.1 tidygraph_1.2.3
[153] yaml_2.3.7 MatrixGenerics_1.12.3
[155] cli_3.6.1 BayesFactor_0.9.12-4.4
[157] stats4_4.3.1 webshot_0.5.5
[159] leiden_0.4.3 lifecycle_1.0.3
[161] uwot_0.1.16 Biobase_2.60.0
[163] mvtnorm_1.2-2 backports_1.4.1
[165] timechange_0.2.0 gtable_0.3.3
[167] rjson_0.2.21 ggridges_0.5.4
[169] progressr_0.14.0 parallel_4.3.1
[171] ape_5.7-1 jsonlite_1.8.7
[173] bitops_1.0-7 bit64_4.0.5
[175] Rtsne_0.16 yulab.utils_0.0.7
[177] spatstat.utils_3.0-3 RcppParallel_5.1.7
[179] formattable_0.2.1 highr_0.10
[181] jquerylib_0.1.4 R.utils_2.12.2
[183] lazyeval_0.2.2 shiny_1.7.5
[185] htmltools_0.5.6 data.tree_1.0.0
[187] glue_1.6.2 SymSim_0.0.0.9000
[189] XVector_0.40.0 RCurl_1.98-1.12
[191] treeio_1.25.3 rprojroot_2.0.3
[193] mclust_6.0.0 ks_1.14.1
[195] mnormt_2.1.1 gridExtra_2.3
[197] igraph_1.5.1 R6_2.5.1
[199] SingleCellExperiment_1.22.0 labeling_0.4.2
[201] cluster_2.1.4 aplot_0.2.0
[203] GenomeInfoDb_1.36.1 plotrix_3.8-2
[205] DelayedArray_0.26.7 tidyselect_1.2.0
[207] vipor_0.4.5 maps_3.4.1
[209] ggforce_0.4.1.9000 xml2_1.3.5
[211] statsExpressions_1.5.1 rsvd_1.0.5
[213] munsell_0.5.0 KernSmooth_2.23-22
[215] optimParallel_1.0-2 data.table_1.14.8
[217] htmlwidgets_1.6.2 ComplexHeatmap_2.16.0
[219] ggmin_0.0.0.9000 rlang_1.1.1
[221] clusterGeneration_1.3.7 spatstat.sparse_3.0-2
[223] spatstat.explore_3.2-1 remotes_2.4.2.1
[225] phangorn_2.11.1 fansi_1.0.4
[227] beeswarm_0.4.0